宝石-溴氟烯烃的光催化E→Z异构化:β-氟苯乙烯衍生物的立体选择性合成。
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
已实现了β-氟苯乙烯衍生物的立体选择性合成。采用具有高三重态能的红外光催化剂,实现了含各种邻位取代基的宝石溴氟烯基苯的选择性异构化。随后的一锅过渡金属(TM)催化反应使以立体选择方式的锅经济合成单氟烯烃成为可能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
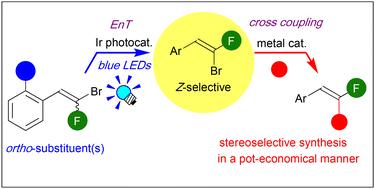
Photocatalytic E → Z isomerization of gem-bromofluoroalkenes: stereoselective synthesis of β-fluorostyrene derivatives†
Stereoselective synthesis of β-fluorostyrene derivatives has been achieved. Selective isomerization of gem-bromofluoroalkenyl benzenes bearing various ortho-substituents is enabled by using Ir photocatalysts with high triplet energy. Subsequent one-pot transition-metal (TM)-catalyzed reactions enable pot-economical synthesis of monofluoroalkenes in a stereoselective manner.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


