皮肤表皮的代谢重新布线驱动对致癌突变的耐受性
IF 19.1
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
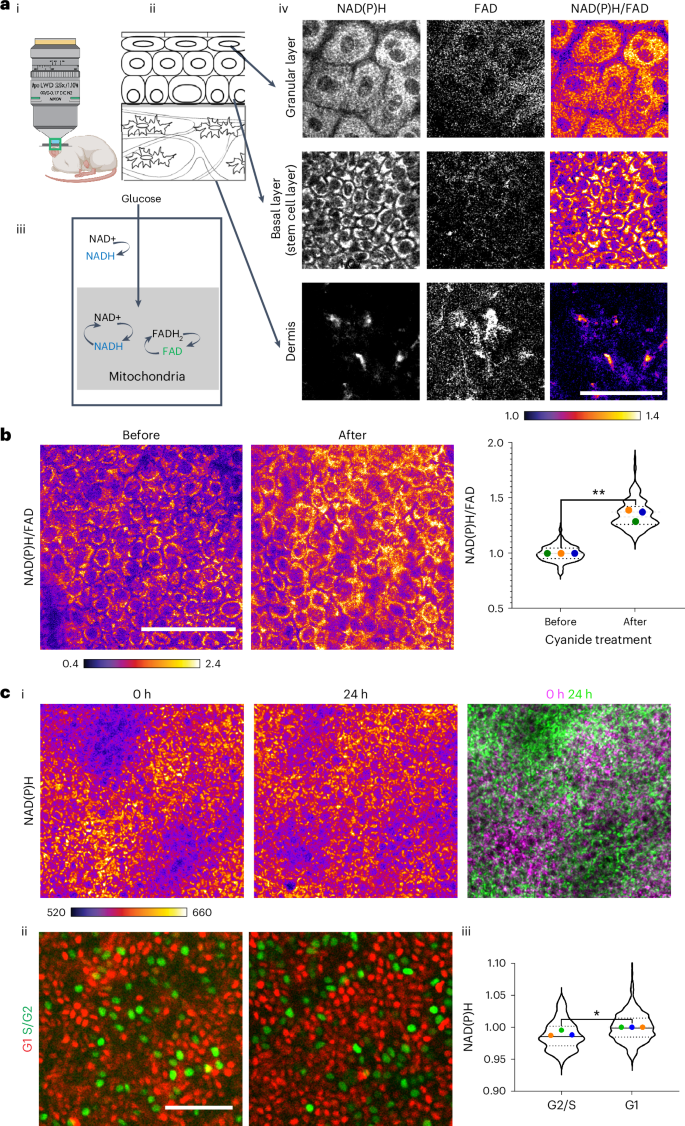
皮肤上皮干细胞可纠正由致癌突变引起的异常。癌基因触发不同的上皮耐受策略;虽然野生型细胞优于β-连环蛋白功能获得(βcatGOF)细胞,但HrasG12V细胞优于野生型细胞。在这里,我们询问野生型干细胞与突变细胞界面并驱动不同的细胞竞争结果时代谢状态如何变化。通过对同一小鼠单细胞分辨率的内源性氧化还原比(NAD(P)H/FAD)随时间的跟踪,我们发现βcatGOF和HrasG12V突变与野生型表皮干细胞结合时,导致氧化还原比迅速下降,表明细胞氧化还原更多。然而,由此产生的氧化还原差异在β类gof中持续存在,而在hrasg12v模型中迅速变平。利用13C液相色谱-串联质谱分析,我们发现βcatGOF和HrasG12V突变体表皮通过氧化三羧酸循环增加了葡萄糖的分数贡献。二甲双胍是一种细胞质氧化还原修饰剂,可以抑制下游突变表型,逆转两种突变模型的细胞竞争结果。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Metabolic rewiring in skin epidermis drives tolerance to oncogenic mutations
Skin epithelial stem cells correct aberrancies induced by oncogenic mutations. Oncogenes invoke different strategies of epithelial tolerance; while wild-type cells outcompete β-catenin-gain-of-function (βcatGOF) cells, HrasG12V cells outcompete wild-type cells. Here we ask how metabolic states change as wild-type stem cells interface with mutant cells and drive different cell-competition outcomes. By tracking the endogenous redox ratio (NAD(P)H/FAD) with single-cell resolution in the same mouse over time, we discover that βcatGOF and HrasG12V mutations, when interfaced with wild-type epidermal stem cells, lead to a rapid drop in redox ratios, indicating more oxidized cellular redox. However, the resultant redox differential persists through time in βcatGOF, whereas it is flattened rapidly in the HrasG12Vmodel. Using 13C liquid chromatography–tandem mass spectrometry, we find that the βcatGOF and HrasG12V mutant epidermis increase the fractional contribution of glucose through the oxidative tricarboxylic acid cycle. Treatment with metformin, a modifier of cytosolic redox, inhibits downstream mutant phenotypes and reverses cell-competition outcomes of both mutant models. Hemalatha et al. track the endogenous redox ratio (NAD(P)H/FAD) with live imaging in the mouse skin to study the interface of wild-type stem cells with oncogenic mutant cells. They find that maintaining a robust redox ratio is key to clonal prevalence.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


