与环境细颗粒物暴露相关的溶甘油磷脂代谢改变:对促动脉粥样硬化作用的见解
IF 7.3
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
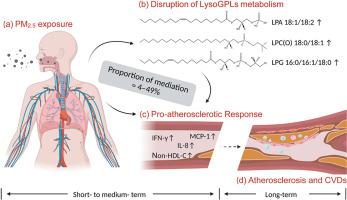
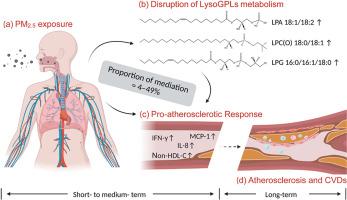
连接环境细颗粒物(PM2.5)诱导的初始不利影响与动脉粥样硬化性心血管疾病发展的生物学途径尚不完全清楚。我们假设溶甘油磷脂(LysoGPLs)是暴露于PM2.5诱导的动脉粥样硬化的关键介质。本研究探讨了PM2.5暴露下溶糖甘氨酸的变化,以及在PM2.5暴露下溶糖甘氨酸的促动脉粥样硬化作用中的中介作用。在这项纵向面板研究中,在2013年至2015年期间,对来自中国北京的110名年龄在50-65岁之间的成年人进行了随访。利用靶向代谢组学分析,对579份血浆样本中5个亚类的18种LysoGPLs进行了量化。在一个监测站监测PM2.5的每日质量浓度。我们使用线性混合效应模型来估计LysoGPLs对PM2.5暴露的反应。随后,进行了中介分析,以研究溶解gpls在pm2.5相关的非高密度脂蛋白-胆固醇(Non-HDL-C)变化中的中介作用,非高密度脂蛋白-胆固醇是一种促动脉粥样硬化载脂蛋白b -含脂蛋白的生物标志物,以及各种炎症生物标志物,包括白细胞介素(IL)-8、单核细胞化学引诱蛋白-1 (MCP-1)、可溶性CD40配体和干扰素(IFN)-γ。中短期(1-30天)PM2.5暴露与6种溶血磷脂酸(LPAs)、3种溶血烷基磷脂酰胆碱[LPC(O)s]和3种溶血磷脂酰甘油(lpg)显著升高,2种溶血磷脂酸和1种溶血磷脂酰丝氨酸(LysoPS)显著降低相关,最大变化幅度分别为0.5-2.1%、0.8-2.1%、1.9-3.0%、-1.4 -3.7%和-8.0%。此外,在30天内,LPA 18:1/18:2、LPC(O) 18:0/18:1和LPG 16:0/16:1/18:0水平的升高显著介导了pm2.5相关的非hdl - c(18-49%)、IL-8(9-24%)、MCP-1(12-26%)和IFN-γ(4-12%)的升高。综上所述,中短期PM2.5暴露与LysoGPLs代谢改变有关,从而介导了PM2.5相关的促动脉粥样硬化反应。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Lysoglycerophospholipid metabolism alterations associated with ambient fine particulate matter exposure: Insights into the pro-atherosclerotic effects
The biological pathways connecting ambient fine particulate matter (PM2.5)-induced initial adverse effects to the development of atherosclerotic cardiovascular diseases are not fully understood. We hypothesize that lysoglycerophospholipids (LysoGPLs) are pivotal mediators of atherosclerosis induced by exposure to PM2.5. This study investigated the changes of LysoGPLs in response to PM2.5 exposure and the mediation role of LysoGPLs in the pro-atherosclerotic effects of PM2.5 exposure. In this longitudinal panel study, 110 adults aged 50–65 years from Beijing, China, were followed between 2013 and 2015. Targeted metabolomics analyses were utilized to quantify 18 LysoGPLs from five subclasses in 579 plasma samples. Daily PM2.5 mass concentration was monitored at a station. We used linear mixed-effect models to estimate the responses of LysoGPLs to PM2.5 exposure. Subsequently, mediation analyses were conducted to investigate the mediating role of LysoGPLs in PM2.5-associated changes in non-high density lipoprotein-cholesterol (Non-HDL-C), a biomarker for pro-atherosclerotic apolipoprotein B-containing lipoproteins, and various inflammatory biomarkers, including interleukin (IL)-8, monocyte chemoattractant protein-1 (MCP-1), soluble CD40 ligand, and interferon (IFN)-γ. Short-to medium-term (1–30 days) PM2.5 exposure was associated with significant increases in six lysophosphatidic acids (LPAs), three lysoalkylphosphatidylcholines [LPC(O)s], and three lysophosphatidylglycerols (LPGs), as well as decreases in two LPAs and one lysophosphatidylserine (LysoPS), with maximus changes of 0.5–2.1%, 0.8–2.1%, 1.9–3.0%, −1.4–3.7%, and −8.0%, respectively. Furthermore, the elevated levels of LPA 18:1/18:2, LPC(O) 18:0/18:1, and LPG 16:0/16:1/18:0 significantly mediated the PM2.5-associated increase in Non-HDL-C (18–49%), IL-8 (9–24%), MCP-1 (12–26%), and IFN-γ (4–12%) over 30 days. In conclusion, short-to medium-term PM2.5 exposure was associated with altered metabolism of LysoGPLs, which mediated the PM2.5-associated pro-atherosclerotic response.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Environmental Pollution
环境科学-环境科学
CiteScore
16.00
自引率
6.70%
发文量
2082
审稿时长
2.9 months
期刊介绍:
Environmental Pollution is an international peer-reviewed journal that publishes high-quality research papers and review articles covering all aspects of environmental pollution and its impacts on ecosystems and human health.
Subject areas include, but are not limited to:
• Sources and occurrences of pollutants that are clearly defined and measured in environmental compartments, food and food-related items, and human bodies;
• Interlinks between contaminant exposure and biological, ecological, and human health effects, including those of climate change;
• Contaminants of emerging concerns (including but not limited to antibiotic resistant microorganisms or genes, microplastics/nanoplastics, electronic wastes, light, and noise) and/or their biological, ecological, or human health effects;
• Laboratory and field studies on the remediation/mitigation of environmental pollution via new techniques and with clear links to biological, ecological, or human health effects;
• Modeling of pollution processes, patterns, or trends that is of clear environmental and/or human health interest;
• New techniques that measure and examine environmental occurrences, transport, behavior, and effects of pollutants within the environment or the laboratory, provided that they can be clearly used to address problems within regional or global environmental compartments.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


