重新定义动脉粥样硬化中内皮到间质转化:信号通路和前瞻性靶向策略
IF 11.4
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
在致病性条件下内皮细胞(ECs)生物学功能的改变导致间充质基质细胞(MSCs)标记物的表达,被定义为内皮到间充质转化(EndMT)。动脉粥样硬化(AS)发病不明显,进展缓慢,是各种动脉粥样硬化性心血管疾病(ASCVD)的潜在诱因。作为AS的“启动者”,EndMT通过触发AS,诱导ASCVD如冠心病(CHD)和缺血性脑血管病(ICD)的进展,并伴有严重的临床并发症如心肌梗死(MI)和脑卒中。深入研究EndMT的病理机制,寻找潜在的靶向治疗策略,对于预防和治疗延迟性EndMT相关的ascvd具有重要的研究价值。尽管以往的研究已经逐渐揭示了EndMT的复杂性及其由血管微环境因素改变引发的致病性,但关于EndMT在AS进展中的最新致病作用、靶向治疗策略及其未来研究方向的系统描述却很少。综述的目的我们的目的是为新的研究者提供全面的关于AS EndMT的知识。本文综述了该领域的最新研究进展,并为研究EndMT这一具有复杂机制的生物过程提供了理论基础。本综述总结了炎症反应或糖酵解、氧化应激、乳酸或乙酰辅酶a (Ac-CoA)、脂肪酸氧化(FAO)、细胞内铁超载和转录因子(包括ELK1和STAT3)组成的微环境串扰改变的血流动力学,这些串扰调节了EndMT并影响了AS的进展。此外,我们为开发针对这些致病过程的有希望的治疗药物提供了新的范例,并指出了阐明EndMT过程需要解决的有希望的方向和挑战。本文章由计算机程序翻译,如有差异,请以英文原文为准。
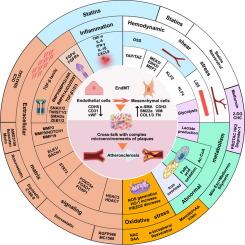
Reconceptualizing Endothelial-to-mesenchymal transition in atherosclerosis: Signaling pathways and prospective targeting strategies
Background
The modification of endothelial cells (ECs) biological function under pathogenic conditions leads to the expression of mesenchymal stromal cells (MSCs) markers, defined as endothelial-to-mesenchymal transition (EndMT). Invisible in onset and slow in progression, atherosclerosis (AS) is a potential contributor to various atherosclerotic cardiovascular diseases (ASCVD). By triggering AS, EndMT, the “initiator” of AS, induces the progression of ASCVD such as coronary atherosclerotic heart disease (CHD) and ischemic cerebrovascular disease (ICD), with serious clinical complications such as myocardial infarction (MI) and stroke. In-depth research of the pathomechanisms of EndMT and identification of potential targeted therapeutic strategies hold considerable research value for the prevention and treatment of ASCVD-associated with delayed EndMT. Although previous studies have progressively unraveled the complexity of EndMT and its pathogenicity triggered by alterations in vascular microenvironmental factors, systematic descriptions of the most recent pathogenic roles of EndMT in the progression of AS, targeted therapeutic strategies, and their future research directions are scarce.Aim of review
We aim to provide new researchers with comprehensive knowledge of EndMT in AS. We exhaustively review the latest research advancements in the field and provide a theoretical basis for investigating EndMT, a biological process with sophisticated mechanisms.Key scientific concepts of review
This review summarized that altered hemodynamics with microenvironmental crosstalk consisting of inflammatory responses or glycolysis, oxidative stress, lactate or acetyl-CoA (Ac-CoA), fatty acid oxidation (FAO), intracellular iron overload, and transcription factors, including ELK1 and STAT3, modulate the EndMT and affect AS progression. In addition, we provide new paradigms for the development of promising therapeutic agents against these disease-causing processes and indicate promising directions and challenges that need to be addressed to elucidate the EndMT process.求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


