Cu-EAB沸石催化剂:NH3-SCR反应中具有优异的SO2抗中毒性能
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
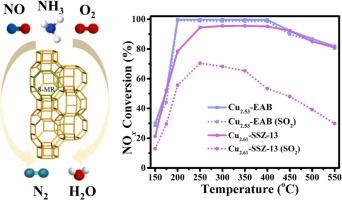
在本研究中,我们合成了具有EAB拓扑结构的Cux-EAB催化剂,用于NOx的NH3-SCR,并首次对其抗SO2中毒性能进行了评价。Cu2.53-EAB催化剂表现出优异的NOx转化率和对N2的选择性,以及对高空速和SO2的耐受性,优于商品Cu2.61-CHA催化剂。在2.53 wt%负荷下,Cu2+主要分布在Cu2.53-EAB结构的双6环和8环中。即使在SO2暴露和多次NH3-SCR循环后,催化剂的性能仍然稳定。200°C和400°C的硫化处理均降低了Cu2.53-EAB和Cu2.61-CHA催化剂的NOx转化率。结果表明,400℃磺化处理对Cu2.53-EAB的NH3-SCR活性影响较小。在200°C和400°C磺化后,H2SO4和CuSO4对活性位点的覆盖是两种催化剂活性降低的主要原因。基于原位DRIFTS分析,提出了NH3-SCR在Cu2.53-EAB上反应的Langmuir-Hinshelwood (L-H)和Eley-Rideal (E-R)混合机理。结果表明,Cu-EAB催化剂是NH3-SCR应用的一种很有前途的替代品,具有更好的SO2抗性和NOx消除能力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Cu-EAB zeolite catalyst: A promising candidate with excellent SO2 poisoning resistance for NH3-SCR reaction
In this work, we synthesized Cux-EAB catalysts with an EAB topology for the NH3-SCR of NOx and evaluated their resistance to SO2 poisoning for the first time. The Cu2.53-EAB catalyst showed superior NOx conversion and selectivity for N2, along with a notable tolerance to high space velocities and SO2, outperforming the commercial Cu2.61-CHA catalyst. This enhanced resistance was attributed to the Cu2 + species formation at the 2.53 wt% loading, which were mainly located in the double 6-rings and 8-rings of the Cu2.53-EAB structure. The catalyst performance was stable even after SO2 exposure and multiple NH3-SCR cycles. Sulfation treatments at both 200 °C and 400 °C reduced the NOx conversion rates of the Cu2.53-EAB and Cu2.61-CHA catalysts. Comparative characterizations before and after sulfation revealed that the NH3-SCR activity of Cu2.53-EAB was less affected by the sulfation treatment at 400 °C. The coverage of active sites by H2SO4 and CuSO4 was identified as the primary cause of activity reduction for both catalysts after sulfation at 200 °C and 400 °C. A hybrid Langmuir-Hinshelwood (L-H) and Eley-Rideal (E-R) mechanism for the NH3-SCR reaction over Cu2.53-EAB was proposed, based on in situ DRIFTS analysis. The results show that the Cu-EAB catalyst is a promising alternative for NH3-SCR applications, offering improved SO2 resistance and NOx elimination capabilities.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


