固态钴、锰卟啉介导H2S/硫醇还原亚硝酸盐的研究
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
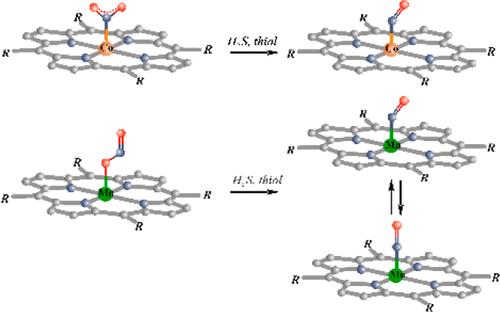
内源性亚硝酸盐还原为亚硝基作为哺乳动物生理缺氧损伤的一种保护机制和一氧化氮的替代来源,参与多种生物活性,正日益受到关注。因此,由金属蛋白介导的这种转化的化学机制引起了相当大的兴趣。本文研究了仿生模型Co(TTP)(NO2) (TTP =中四苯基卟啉钠离子)和Mn(TPP)(ONO) (TPP =中四苯基卟啉钠离子)在升华固体膜中与硫化氢(H2S)和乙硫醇(EtSH)在从77 K到室温的不同温度下的反应。在这两种情况下,配位亚硝酸盐配合物最终分别转化为亚硝基Co(TTP)(NO)和Mn(TPP)(NO);在低温条件下,首次生成了新型六坐标配合物M(Por)(RSH)(亚硝酸盐)。在过量硫醇存在的情况下加热这些薄膜导致两种亚硝基络合物的形成。报道了挥发产物的质谱分析和可能中间体的DFT计算,并讨论了配位亚硝酸盐离子还原的潜在机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Nitrite Reduction with H2S/Thiol Mediated by Cobalt and Manganese Porphyrins in the Solid State
The endogenous reduction of nitrite to nitrosyl is drawing increasing attention as a protective mechanism against hypoxic injury in mammalian physiology and as an alternative source of NO, which is involved in a wide variety of biological activities. Thus, chemical mechanisms for this transformation, which are mediated by metallo proteins, are of considerable interest. The study described here examines the reactions of the biomimetic models Co(TTP)(NO2) (TTP = meso-tetratolylporphyrinato dianion) and Mn(TPP)(ONO) (TPP = meso-tetraphenyl-porphyrinato dianion) in sublimated solid films with hydrogen sulfide (H2S) and with ethanethiol (EtSH) at various temperatures from 77 K to room temperature using in situ infrared and optical spectroscopy. In both cases, the coordinated nitrite complex is eventually converted to the respective nitrosyl Co(TTP)(NO) and Mn(TPP)(NO); however, reaction at low temperature first gave a novel six-coordinate complex M(Por)(RSH)(nitrite). Warming these films in the presence of excess thiol resulted in the formation of the two nitrosyl complexes. Mass spectrometric analysis of volatile products and DFT computations of possible intermediates are reported, and potential mechanisms for reduction of the coordinated nitrite ions are discussed.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


