揭示用于增强电化学CO2还原的离子材料的关键描述符
IF 18.2
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
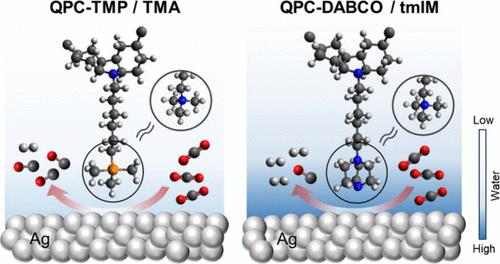
CO2还原反应(CO2RR)电极催化剂表面附近的聚合物离聚体影响其效率;然而,它们的多面性使构效关系的阐明变得复杂。在这里,我们合成了具有不同功能化侧链的聚咔唑基阴离子交换(QPC)离聚体来探索这种关系。综合分析ATR-SEIRAS的理化性质、电化学活性和operando,发现官能团修饰显著影响了本征离聚体的性质,从而影响了Ag催化剂的性质、界面水结构的微环境以及CO2RR的质子化步骤和析氢反应(HER)的反应动力学。值得注意的是,qpc -三甲基膦(TMP)离聚体诱导了良好的界面水结构,具有高比例的强h键水和低Stark调谐斜率,抑制了HER并促进了CO2RR。在膜电极组件中使用QPC-TMP,即使在不同的CO2浓度(100-15%)和高温(28-72°C)下,也能保持较高的CO法拉第效率(>90%)。这些发现表明,可以通过微调离聚体结构来优化催化环境,从而促进高性能CO2RR离聚体的发展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Unveiling Key Descriptors of Ionomer Materials for Enhanced Electrochemical CO2 Reduction
Polymeric ionomers near the catalyst surface of CO2 reduction reaction (CO2RR) electrodes affect their efficiency; however, their multifaceted properties complicate structure–activity relationship elucidation. Here, we synthesized polycarbazole-based anion-exchange (QPC) ionomers bearing varying functionalized side chains to explore this relationship. Comprehensive analysis in physicochemical properties, electrochemical activity, and operando ATR-SEIRAS revealed that functional group modification significantly influenced the intrinsic ionomer properties, thereby affecting the Ag catalyst properties, microenvironments of interfacial water structures, and reaction kinetics of the protonation step for CO2RR and the hydrogen evolution reaction (HER). Notably, the QPC-trimethyl phosphonium (TMP) ionomer induced favorable interfacial water structures, having a high proportion of strong H-bonded water with low Stark tuning slopes, which inhibit HER and promote CO2RR. A high CO Faradaic efficiency (>90%) was maintained using QPC-TMP in a membrane electrode assembly, even under varying CO2 concentrations (100–15%) and elevated temperatures (28–72 °C). These findings suggest that the catalytic environment can be optimized by fine-tuning the ionomer structure, contributing to the advancement of high-performance CO2RR ionomers.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Energy Letters
Energy-Renewable Energy, Sustainability and the Environment
CiteScore
31.20
自引率
5.00%
发文量
469
审稿时长
1 months
期刊介绍:
ACS Energy Letters is a monthly journal that publishes papers reporting new scientific advances in energy research. The journal focuses on topics that are of interest to scientists working in the fundamental and applied sciences. Rapid publication is a central criterion for acceptance, and the journal is known for its quick publication times, with an average of 4-6 weeks from submission to web publication in As Soon As Publishable format.
ACS Energy Letters is ranked as the number one journal in the Web of Science Electrochemistry category. It also ranks within the top 10 journals for Physical Chemistry, Energy & Fuels, and Nanoscience & Nanotechnology.
The journal offers several types of articles, including Letters, Energy Express, Perspectives, Reviews, Editorials, Viewpoints and Energy Focus. Additionally, authors have the option to submit videos that summarize or support the information presented in a Perspective or Review article, which can be highlighted on the journal's website. ACS Energy Letters is abstracted and indexed in Chemical Abstracts Service/SciFinder, EBSCO-summon, PubMed, Web of Science, Scopus and Portico.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


