提高能量密度的Mg电池中动力学生长的氧化钼的层间膨胀
IF 18.9
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
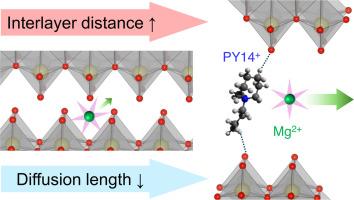
最先进的锂离子电池(LIBs)支持电动汽车(ev)和储能系统(ESS),但其有限的能量密度推动了替代“后锂离子电池”的研究。多价(MV)离子插入化学具有增加可充电电池理论能量密度的潜力,同时利用成熟的锂离子插入宿主。然而,较高的电荷密度会导致氧化物主体的界面和体扩散动力学的实质性障碍,由于它们在硫族化合物中具有最高的氧化还原电位和最大的理论容量,因此处于阴极开发的前沿。在本研究中,我们通过引入有机柱,将三氧化钼(MoO3)的层间间距扩大到12.3 Å,即原始MoO3的180%,设计了镁离子(Mg2+作为mv离子的代表)电池中具有代表性的层状氧化物主体。同时,通过诱导初生颗粒的动力学生长,将MoO3的扩散路径长度减小到商品MoO3的5.8%。原位层间膨胀和动力学生长策略协同作用,增强了界面和固有体扩散动力学,从而使材料的比容量显著提高,达到352 mAh/g,是原始MoO3的2.2倍。这种工程多尺度微结构的协同策略为促进各种mv离子嵌入提供更高能量密度的层状氧化物开辟了新的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Interlayer expansion of kinetically grown molybdenum oxide for Mg batteries with enhanced energy density
The state-of-the-art lithium-ion batteries (LIBs) enabled electric vehicles (EVs) and energy storage systems (ESS), but their limited energy density has driven research into alternative “post-LIBs.” Multivalent (MV)-ion intercalation chemistry holds potential for multiplying the theoretical energy density of rechargeable batteries while utilizing the well-established intercalation hosts for LIBs. However, the higher charge density of MV-ions leads to substantial hindrances in interfacial and bulk diffusion kinetics in oxide hosts, which are at the forefront of cathode development due to their highest redox potential and largest theoretical capacity among the chalcogenides. In this study, we engineer the crystal structure of molybdenum trioxide (MoO3), a representative layered oxide host in magnesium-ion (Mg2+ as a representative MV-ion) batteries, by introducing organic pillars to expand the interlayer spacing to 12.3 Å, i.e., 180 % of pristine MoO3. At the same time, we induced the kinetic growth of the primary particles to reduce the diffusion path lengths to 5.8 % of commercial bulk MoO3. The in-situ inter-layer expansion and kinetic growth strategies work synergistically to enhance the interfacial and intrinsic bulk diffusion kinetics, resulting in a material with a significantly increased specific capacity of 352 mAh/g as a cathode for magnesium-ion batteries, which provides 2.2 times larger capacity than the pristine MoO3. This synergetic strategy of engineering multiscale microstructures may open a new avenue for the facilitation of various MV-ions’ intercalation into the layered oxides that provide higher energy density.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Energy Storage Materials
Materials Science-General Materials Science
CiteScore
33.00
自引率
5.90%
发文量
652
审稿时长
27 days
期刊介绍:
Energy Storage Materials is a global interdisciplinary journal dedicated to sharing scientific and technological advancements in materials and devices for advanced energy storage and related energy conversion, such as in metal-O2 batteries. The journal features comprehensive research articles, including full papers and short communications, as well as authoritative feature articles and reviews by leading experts in the field.
Energy Storage Materials covers a wide range of topics, including the synthesis, fabrication, structure, properties, performance, and technological applications of energy storage materials. Additionally, the journal explores strategies, policies, and developments in the field of energy storage materials and devices for sustainable energy.
Published papers are selected based on their scientific and technological significance, their ability to provide valuable new knowledge, and their relevance to the international research community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


