na2co3改性活性炭负载Cu-Fe氧化物促进低温脱除H2S
IF 9
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
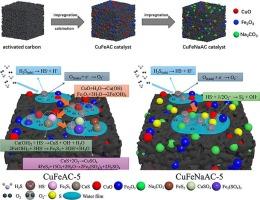
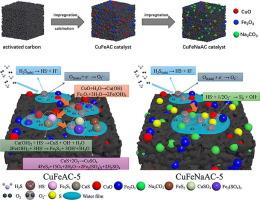
采用浸渍法和煅烧法制备了多种负载在na2co3改性活性炭(CuFeNaAC-X)催化剂上的Cu-Fe氧化物,并对其低温脱硫性能进行了评价。通过优化Cu-Fe的负载量,CuFeNaAC-5的硫容量达到430.09 mg/g,超过了大多数活性炭(AC)催化剂。EPR测试表明,负载Cu-Fe氧化物可以激活氧形成超氧自由基(O2-·),从而促进H2S的催化氧化。XPS测试表明,负载Cu-Fe氧化物后,FeOOH的形成有利于H2S通过链式反应催化氧化成硫(S)。结合EPR和XPS分析,Na2CO3修饰可以调节O2-·的含量,从而指导S的形成,从而显著提高催化剂的脱硫效率。此外,CO2-TPD测试表明,Na2CO3改性促进H2S电离为HS-,从而提高了催化剂的脱硫性能。根据催化剂中硫的分布和表征结果,提出了CuFeNaAC-X可能的脱硫机理。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Cu-Fe oxide supported on Na2CO3-modified activated carbon boost H2S removal at low temperature
A variety of Cu-Fe oxide supported on Na2CO3-modified activated carbon (CuFeNaAC-X) catalysts were prepared via the impregnation and calcination methods and their desulfurization performance was evaluated at low-temperature. By optimizing the loading content of Cu-Fe, the sulfur capacity of the CuFeNaAC-5 reached a maximum of 430.09 mg/g, surpassing most activated carbon (AC) catalysts. EPR testing revealed that the loading of Cu-Fe oxide can activate oxygen to form superoxide radicals (·), thereby promoting the catalytic oxidation of H2S. XPS testing revealed that upon loading of Cu-Fe oxide, the formation of FeOOH can facilitate the catalytic oxidation of H2S to sulfur (S) through a chain reaction. Combining EPR and XPS analyses, Na2CO3 modification modulates the content of ·, directing the formation of S and then significantly improving the catalyst’s desulfurization efficiency. Furthermore, CO2-TPD testing revealed that Na2CO3 modification promotes the ionization of H2S to HS-, thereby enhancing the catalyst’s desulfurization performance. Based on the distribution of sulfur species in used catalysts, and the results of characterizations, a possible desulfurization mechanism of CuFeNaAC-X has been proposed.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


