大麻素受体的新型正构/变构配体:意想不到的药理学概况
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
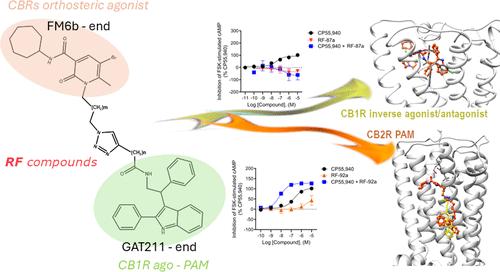
设计双位/双位受体配体作为能够同时与正构和变构结合位点相互作用的化合物,对于增强受体特异性和减少脱靶效应具有重要意义。在这项工作中,我们报道了由CB1R ago阳性变构调节剂(PAM) GAT211与大麻素受体(CBRs)正构激动剂FM6b化学结合而成的一系列新化合物,即RF系列的合成和生物学评价。因此,RF化合物被设计为hCB1R的双位/双位配体,目的是获得更强的hCB1R激动剂或ago- pam,提高受体亚型选择性,减少中枢副作用。出乎意料的是,对hCB1R的体外实验表明,RF化合物是逆激动剂/拮抗剂,与母体化合物FM6b和GAT211相比,表现出不同的特征,此外,两种化合物表现为hCB2R pam。这些新配体功能的不可预测的变化表明大麻素的功能不是简单地预测的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Novel Orthosteric/Allosteric Ligands of Cannabinoid Receptors: An Unexpected Pharmacological Profile
The design of dualsteric/bitopic receptor ligands as compounds capable of simultaneously interacting with both the orthosteric and an allosteric binding site has gained importance to achieve enhanced receptor specificity and minimize off-target effects. In this work, we reported the synthesis and biological evaluation of a new series of compounds, namely, the RF series, obtained by chemically combining the CB1R ago-positive allosteric modulators (PAM) GAT211 with the cannabinoid receptors (CBRs) orthosteric agonist FM6b. Therefore, RF compounds were designed as dualsteric/bitopic ligands for hCB1R with the aim of obtaining stronger hCB1R agonists or ago-PAMs, with improved receptor subtype selectivity and reduction of central side effects. Unexpectedly, in vitro assays on hCB1R indicated RF compounds were inverse agonists/antagonists, exhibiting different profiles compared to those of parent compounds FM6b and GAT211 and, furthermore, two compounds behaved as hCB2R PAMs. The unpredictable change in the function of these new ligands suggests that the function of cannabinoids is not simply predicted.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


