快速和绿色阴离子辅助的机械化学肽环化
IF 7.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
描述了一种新的机械化学方法,用于氯化物模板头到尾的五肽和六肽大环化。这种简单的方法允许无溶剂制备环肽,其产率与基于溶液的方法相当,而不需要高度稀释反应混合物,并且显著减少反应时间和有机废物量。本文章由计算机程序翻译,如有差异,请以英文原文为准。
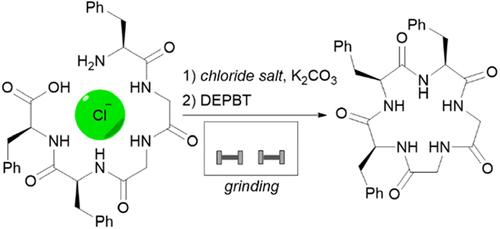
Rapid and Green Anion-Assisted Mechanochemical Peptide Cyclization
A novel mechanochemical approach is described for chloride-templated head-to-tail macrocyclization of a pentapeptide and a hexapeptide. This straightforward method allows the solvent-free preparation of cyclopeptides with yields comparable to solution-based approaches without the need for high dilution of the reaction mixture and with significantly reduced reaction times and organic waste amount.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


