伴侣介导的线粒体输入受体TOM70的插入可防止饮食引起的肥胖
IF 17.3
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
线粒体外膜(OMM)蛋白与细胞质和其他细胞器(包括内质网)进行通讯。这种交流在产热脂肪细胞中很重要,可以增加控制体温和体重的能量消耗。然而,OMM蛋白插入的调控机制尚不清楚。在这里,应激诱导的细胞质伴侣PPID(肽酰脯氨酸异构酶D/亲环蛋白40/Cyp40)驱动线粒体输入受体TOM70的OMM插入,TOM70调节肥胖小鼠的体温和体重,以及棕色脂肪细胞的呼吸/产热功能。PPID PPIase活性和c端四肽重复序列对TOM70核心和c尾结构域具有特异性,有助于OMM插入。我们的研究结果表明,内质网应激激活的伴侣蛋白通过选择性OMM蛋白插入机制在控制能量代谢方面发挥了前所未有的作用,这对适应低温和高热量饮食具有重要意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。
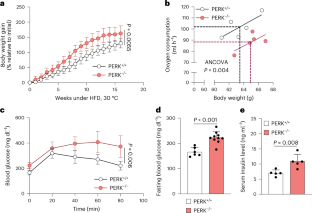

Chaperone-mediated insertion of mitochondrial import receptor TOM70 protects against diet-induced obesity
Outer mitochondrial membrane (OMM) proteins communicate with the cytosol and other organelles, including the endoplasmic reticulum. This communication is important in thermogenic adipocytes to increase the energy expenditure that controls body temperature and weight. However, the regulatory mechanisms of OMM protein insertion are poorly understood. Here the stress-induced cytosolic chaperone PPID (peptidyl–prolyl isomerase D/cyclophilin 40/Cyp40) drives OMM insertion of the mitochondrial import receptor TOM70 that regulates body temperature and weight in obese mice, and respiratory/thermogenic function in brown adipocytes. PPID PPIase activity and C-terminal tetratricopeptide repeats, which show specificity towards TOM70 core and C-tail domains, facilitate OMM insertion. Our results provide an unprecedented role for endoplasmic-reticulum-stress-activated chaperones in controlling energy metabolism through a selective OMM protein insertion mechanism with implications in adaptation to cold temperatures and high-calorie diets. Latorre-Muro et al. show that the cytosolic chaperone PPID drives insertion of the mitochondrial import receptor TOM70 into the mitochondrial outer membrane, thereby regulating body temperature, glucose homeostasis and body weight in obese mice.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


