纳米点包裹的自噬体可作为个体化癌症疫苗
IF 34.9
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
自噬体癌症疫苗可促进多种肿瘤抗原的交叉呈递并诱导交叉反应性T细胞反应。然而,迄今为止,由于自噬体一旦形成,迅速与溶酶体融合,不易从细胞中逃逸,尚无获得高免疫原性的自噬体癌疫苗的有效方法。在这里,我们报道了一个功能性的Ti2NX纳米点,它可以覆盖自噬体膜脂磷脂酰肌醇-4-磷酸,阻断自噬体与溶酶体的融合,并在肿瘤中产生稳定的纳米点包裹的自噬体。形成的纳米点包裹的自噬体可以从癌细胞逃到淋巴结,在那里它们激活肿瘤特异性T细胞。我们表明,我们的方法减少了肿瘤负担,并为治愈小鼠提供了长期的免疫监视保护。这项工作为在体内直接形成基于自噬体的个性化癌症疫苗提供了一种方法,为肿瘤治疗提供了一种有希望的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
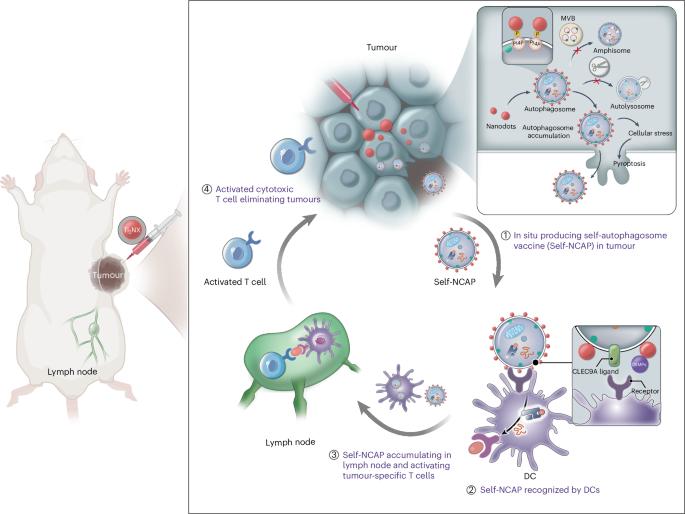
Autophagosomes coated in situ with nanodots act as personalized cancer vaccines
Autophagosome cancer vaccines can promote cross-presentation of multiple tumour antigens and induce cross-reactive T cell responses. However, so far, there is no effective method for obtaining a highly immunogenic autophagosomal cancer vaccine because autophagosomes, once formed, quickly fuse with lysosomes and cannot easily escape from cells. Here we report a functional Ti2NX nanodot that caps the autophagosome membrane lipid phosphatidylinositol-4-phosphate, blocking the fusion of autophagosomes with lysosomes and producing stable nanodot-coated autophagosomes in tumours. The formed nanodot-coated autophagosomes can escape from cancer cells to lymph nodes, where they activate tumour-specific T cells. We show that our approach reduces tumour burden and provide long-term immune surveillance protection for cured mice. This work provides a method for the direct formation of personalized autophagosome-based cancer vaccines in vivo, offering a promising strategy for tumour treatment. Cancer vaccine is a powerful cancer immunotherapy strategy. Here autophagosomes coated with nanodots in situ in tumours serve as personalized cancer vaccines, effectively activating anti-cancer immune responses in vivo.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature nanotechnology
工程技术-材料科学:综合
CiteScore
59.70
自引率
0.80%
发文量
196
审稿时长
4-8 weeks
期刊介绍:
Nature Nanotechnology is a prestigious journal that publishes high-quality papers in various areas of nanoscience and nanotechnology. The journal focuses on the design, characterization, and production of structures, devices, and systems that manipulate and control materials at atomic, molecular, and macromolecular scales. It encompasses both bottom-up and top-down approaches, as well as their combinations.
Furthermore, Nature Nanotechnology fosters the exchange of ideas among researchers from diverse disciplines such as chemistry, physics, material science, biomedical research, engineering, and more. It promotes collaboration at the forefront of this multidisciplinary field. The journal covers a wide range of topics, from fundamental research in physics, chemistry, and biology, including computational work and simulations, to the development of innovative devices and technologies for various industrial sectors such as information technology, medicine, manufacturing, high-performance materials, energy, and environmental technologies. It includes coverage of organic, inorganic, and hybrid materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


