三(2-氯乙基)磷酸通过激活髓质和髓外红细胞生成导致小鼠循环红细胞不平衡
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
三(2-氯乙基)磷酸(TCEP)是一种普遍存在的有机磷阻燃剂,已在各种环境基质和人体血液样本中被发现,引起了人们对其血液毒性的警惕,这一主题尚未得到彻底的研究。红细胞(rbc)或红细胞是外周血中主要的细胞类型,对维持生理健康至关重要。本研究采用口服灌胃的方法来研究TCEP暴露对小鼠红细胞计数的影响,并阐明其潜在机制。结果显示,tcep暴露后循环红细胞计数显著增加,同时增加真性红细胞增多症(PV)的风险。TCEP暴露刺激了髓质发育的所有阶段的红细胞生成,包括造血干细胞向红祖细胞的分化、红细胞发育的进展和红细胞的成熟。此外,TCEP增强了脾和肝的髓外红细胞生成。随后的生物信息学分析表明,tcepa诱导的红细胞生成归因于p53下调。因此,这些发现表明,TCEP通过增强髓质和髓外红细胞生成,破坏红细胞介导的血液稳态,导致血液平衡的改变。本文章由计算机程序翻译,如有差异,请以英文原文为准。
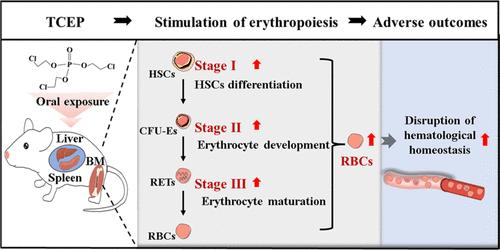
Tris(2-chloroethyl) Phosphate Leads to Unbalanced Circulating Erythrocyte in Mice by Activating both Medullary and Extramedullary Erythropoiesis
Tris(2-chloroethyl) phosphate (TCEP), a prevalent organophosphorus flame retardant, has been identified in various environmental matrices and human blood samples, provoking alarm regarding its hematological toxicity, a subject that has not been thoroughly investigated. Red blood cells (RBCs), or erythrocytes, are the predominant cell type in peripheral blood and are crucial for the maintenance of physiological health. This investigation employed oral gavage to examine the effects of TCEP exposure on erythrocyte counts in mice and to clarify the underlying mechanisms. The results demonstrated a marked increase in circulating RBC counts post-TCEP exposure, concomitantly heightening the risk of polycythemia vera (PV). TCEP exposure stimulated erythropoiesis across all stages of medullary development, including the differentiation of hematopoietic stem cells into erythroid progenitors, the progression of erythrocyte development, and the maturation of erythrocyte. Moreover, TCEP potentiated extramedullary erythropoiesis in the spleen and liver. Subsequent bioinformatics analysis implied that TCEP-induced erythropoiesis was attributed to p53 downregulation. Thus, these findings indicate that TCEP disrupts erythrocyte-mediated hematological homeostasis through the enhancement of both medullary and extramedullary erythropoiesis, leading to the alteration of hematological equilibrium.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


