蛋白质加冕诱导的癌症分期依赖的多水平细胞毒性:血管类器官的全人源化研究
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
蛋白质冠效应是指血液中的纳米材料被血清蛋白包裹的现象,然而蛋白质冠状纳米材料如何与血管相互作用及其毒性影响仍然知之甚少。在这项研究中,我们通过整合血管类器官和患者来源血清的全人源化试验来研究蛋白质冠状病毒相关的血管毒性。首先,我们筛选了各种纳米材料,以了解包括尺寸、形态、疏水性、表面电荷和手性依赖性蛋白电晕差异在内的参数如何影响它们被血管类器官吸收。对于在血管摄取方面表现出实质性差异的纳米材料,使用无标记质谱分析了它们的蛋白质冠。我们的研究结果揭示了癌症分期相关的细胞骨架成分参与介导细胞的优先摄取,包括内皮细胞和壁细胞。此外,还进行了转录组研究来阐明纳米材料的影响。我们证实,蛋白质冠状纳米材料在转录和翻译水平上引发重塑,分别影响PI3K-Akt/Hippo/Wnt等通路和无膜细胞器完整性。我们的研究进一步表明,患者来源的蛋白质冠状纳米材料的重塑潜力可以与抗血管生成疗法协同作用,以改善结果。我们期望本研究能为未来纳米药物的安全使用提供指导。本文章由计算机程序翻译,如有差异,请以英文原文为准。
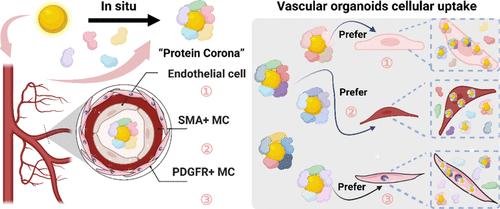
Protein Coronation-Induced Cancer Staging-Dependent Multilevel Cytotoxicity: An All-Humanized Study in Blood Vessel Organoids
The protein corona effect refers to the phenomenon wherein nanomaterials in the bloodstream are coated by serum proteins, yet how protein coronated nanomaterials interact with blood vessels and its toxicity implications remain poorly understood. In this study, we investigated protein corona-related vessel toxicity by using an all-humanized assay integrating blood vessel organoids and patient-derived serum. Initially, we screened various nanomaterials to discern how parameters including size, morphology, hydrophobicity, surface charge, and chirality-dependent protein corona difference influence their uptake by vessel organoids. For nanomaterials showing substantial differences in vessel uptake, their protein corona was analyzed by using label-free mass spectra. Our findings revealed the involvement of cancer staging-related cytoskeleton components in mediating preferential uptake by cells, including endothelial and mural cells. Additionally, a transcriptome study was conducted to elucidate the influence of nanomaterials. We confirmed that protein coronated nanomaterials provoke remodeling at both transcriptional and translational levels, impacting pathways such as PI3K-Akt/Hippo/Wnt, and membraneless organelle integrity, respectively. Our study further demonstrated that the remodeling potential of patient-derived protein coronated nanomaterials can be harnessed to synergize with antiangiogenesis therapeutics to improve the outcomes. We anticipate that this study will provide guidance for the safe use of nanomedicine in the future.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


