结合营养限制和联合治疗的功能化微球平台对抗细菌感染
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
耐多药(MDR)细菌感染的日益流行已成为一项严重的全球健康危机,破坏了传统抗生素疗法的疗效。这一紧迫挑战要求制定创新战略,以对抗耐多药病原体。多功能给药系统的进展为减少或根除耐多药耐药细菌提供了有希望的解决方案。细菌的生长需要必需的营养物质,受此启发,制备了包被ph响应性聚多巴胺(PDA)的核-壳多孔聚乳酸-羟基乙酸(PLGA)微球来改善递送,从而通过营养限制和联合治疗提高了疗效。PDA在环境中螯合铁离子,阻止细菌吸收铁,从而抑制细菌的生长和增殖。随后,从多孔PLGA核心释放的抗生素利福平和多粘菌素B通过破坏细菌的内外膜结构来加速细菌的消灭。这种多功能微球平台在4小时内清除了99%的鼠伤寒沙门菌,并在小鼠致死性肠道感染模型中显示出更高的效率。这些发现提供了一种整合细菌营养限制和抗生素杀伤的药物传递系统,突出了靶向细菌铁调节作为开发新的抗多药耐药细菌感染的抗菌药物传递系统策略的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
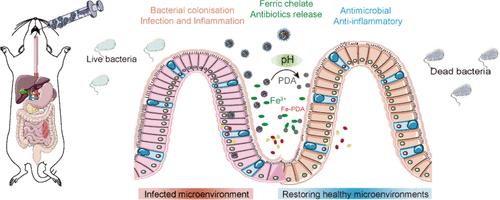
Functionalized Microsphere Platform Combining Nutrient Restriction and Combination Therapy to Combat Bacterial Infections
The escalating prevalence of multidrug-resistant (MDR) bacterial infections has emerged as a critical global health crisis, undermining the efficacy of conventional antibiotic therapies. This pressing challenge necessitates the development of innovative strategies to combat MDR pathogens. Advances in multifunctional drug delivery systems offer promising solutions to reduce or eradicate MDR bacteria. Inspired by the fact that the growth of bacteria requires essential nutrients, core–shell porous poly(lactic-co-glycolic acid) (PLGA) microspheres coated with pH-responsive polydopamine (PDA) were fabricated to improve delivery, resulting in enhanced efficacy through nutrient restriction and combination therapy. The PDA chelates iron ions in the environment, preventing bacteria from absorbing iron and thus suppressing their growth and proliferation. Subsequently, the released antibiotics from the porous PLGA core, rifampicin and polymyxin B, accelerate bacterial eradication by disrupting their inner and outer membrane structures. Such a multifunctional microsphere platform clears 99% Salmonella Typhimurium in 4 h and shows increased efficiency in a lethal intestinal infection model in mice. These findings provide a drug delivery system that integrates bacterial nutrient restriction and antibiotic killing, highlighting the potential of targeting bacterial iron regulation as a strategy for developing new antimicrobial delivery systems to address MDR bacterial infections.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


