Parkin通过调节PD-1/PD-L1信号传导调节肝癌微环境
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
帕金森介导的线粒体自噬是清除受损线粒体所必需的,它抑制肿瘤的发展。线粒体自噬在调节肿瘤免疫中的作用越来越清楚,但其潜在的机制仍然知之甚少。目的探讨Parkin在四氯化碳(CCl4)诱导的肝肿瘤免疫微环境中的作用。方法采用单细胞RNA测序、Western blot、免疫荧光和免疫共沉淀法验证Parkin通过改变PD-1表达影响肿瘤微环境的机制。结果我们的数据显示,Park2-/-小鼠表现出严重的肝损伤和恶性肿瘤的增加。肝脏肿瘤T淋巴细胞单细胞RNA测序分析显示,Park2-/-小鼠的细胞毒性CD8+ T细胞(Gzmb/Ifng/Fasl)数量显著减少,耗竭CD8+ T细胞(Pdcd1/Lag3/Tigit/Havcr2/Ctla4)数量显著增加,提示存在免疫抑制微环境。骨髓源性细胞的单细胞RNA测序分析也显示Park2-/-小鼠中m2样巨噬细胞的增加。通过定量蛋白质组学分析发现,两组差异蛋白的表达主要定位于质膜和细胞外,包括PD-1、MHC-Ⅰ分子等,且主要与PD-1和抗原递呈途径相关。它可能损害帕金森氏症患者的抗肿瘤免疫反应。帕金缺乏导致肝脏自噬水平降低,形成免疫抑制微环境,促进肝癌的发生。结论Parkin作为E3泛素连接酶,可诱导肝癌组织中PD-1的泛素化和降解,并通过PD-1/PD-L1信号通路影响抗肿瘤免疫。因此,通过重新引入Parkin或增强线粒体自噬来重塑肿瘤微环境可以激活抗肿瘤免疫反应,提高免疫治疗效果,这可能是治疗HCC的一种有希望的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
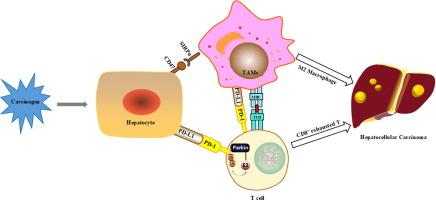
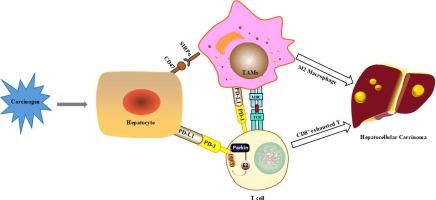
Parkin modulates the hepatocellular carcinoma microenvironment by regulating PD-1/PD-L1 signalling
Introduction
Parkin-mediated mitophagy is essential for clearing damaged mitochondria, and it inhibits tumour development. The role of mitophagy in modulating tumour immunity is becoming clearer, but the underlying mechanism is still poorly understood.
Objective
This study was designed to examine the role of Parkin in the immune microenvironment of liver tumours induced by carbon tetrachloride (CCl4).
Methods
Single-cell RNA sequencing analysis, Western blot, immunofluorescence and co-immunoprecipitation were used to verify the mechanism of Parkin affecting the tumour microenvironment by altering the expression of PD-1.
Results
Our data revealed that Park2-/- mice showed severe liver damage and increased malignancy. Single-cell RNA sequencing analysis of T lymphocytes in liver tumours showed that the number of cytotoxic CD8+ T cells (Gzmb/Ifng/Fasl) was significantly decreased and the number of exhausted CD8+ T cells (Pdcd1/Lag3/Tigit/Havcr2/Ctla4) was significantly increased in Park2-/- mice, indicating the immune suppressive microenvironment. Single-cell RNA sequencing analysis of myeloid-derived cells also displayed the increase of M2-like macrophages in Park2-/- mice. Through quantitative proteomic analysis, it was found that the differential protein expression between the two groups mainly localized in the plasma membrane and extracellular, including PD-1, MHC-Ⅰ molecules etc., and was mainly associated with PD-1 and antigen presentation pathways. It could impair the antitumour immune response with Parkin deficiency. Parkin deficiency leads to the decrease of hepatic mitophagy levels and the formation of an immune suppressive microenvironment, which promotes the tumourigenesis of liver cancer.
Conclusion
As an E3 ubiquitin ligase, Parkin induces the ubiquitination and degradation of PD-1 in liver cancer and could influence antitumour immunity through the PD-1/PD-L1 signalling pathway. Thus, remodeling the tumour microenvironment through the reintroduction of Parkin or enhancement of mitophagy could activate the anti-tumour immune response and improve the immunotherapy efficacy, which may be a promising strategy for the treatment of HCC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


