ampk调节的甘油排泄维持还原性和能量应激之间的代谢串扰
IF 17.3
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
葡萄糖代谢已被广泛研究,但葡萄糖衍生的排泄甘油的作用仍不清楚。本研究表明,缺氧诱导NADH积累,促进甘油排泄,这一途径持续消耗NADH,从而减弱其积累和还原性应激。醛缩酶B通过与甘油3-磷酸脱氢酶GPD1和GPD1L形成络合物参与甘油的生物合成。阻断GPD1、GPD1L或甘油3-磷酸磷酸酶可加重体内缺氧条件下的还原性应激,抑制细胞增殖和肿瘤生长。这些酶的过度表达会增加甘油的分泌,但在缺氧和能量应激引起的肿瘤增殖下仍会降低细胞活力。AMPK灭活醛缩酶B以减缓甘油合成,从而耗散ATP,缓解NADH积累引起的能量危机。因此,甘油的生物合成/排泄调节着还原性应激和能量应激之间的平衡。此外,这种调节模式似乎在减压驱动的转化中普遍存在,增强了我们对代谢复杂性的理解,并指导肿瘤治疗。本文章由计算机程序翻译,如有差异,请以英文原文为准。
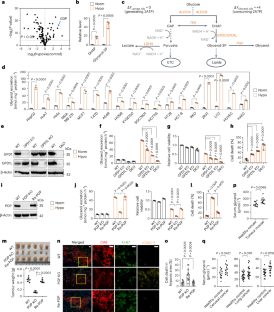

AMPK-regulated glycerol excretion maintains metabolic crosstalk between reductive and energetic stress
Glucose metabolism has been studied extensively, but the role of glucose-derived excretory glycerol remains unclear. Here we show that hypoxia induces NADH accumulation to promote glycerol excretion and this pathway consumes NADH continuously, thus attenuating its accumulation and reductive stress. Aldolase B accounts for glycerol biosynthesis by forming a complex with glycerol 3-phosphate dehydrogenases GPD1 and GPD1L. Blocking GPD1, GPD1L or glycerol 3-phosphate phosphatase exacerbates reductive stress and suppresses cell proliferation under hypoxia and tumour growth in vivo. Overexpression of these enzymes increases glycerol excretion but still reduces cell viability under hypoxia and tumour proliferation due to energy stress. AMPK inactivates aldolase B to mitigate glycerol synthesis that dissipates ATP, alleviating NADH accumulation-induced energy crisis. Therefore, glycerol biosynthesis/excretion regulates the trade-off between reductive stress and energy stress. Moreover, this mode of regulation seems to be prevalent in reductive stress-driven transformations, enhancing our understanding of the metabolic complexity and guiding tumour treatment. Zhai, Yang et al. report a central role for AMPK in regulating aldolase B-mediated glycerol synthesis and excretion under hypoxia as a mechanism to balance the trade-off between reductive and energy stress during tumour growth.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


