同源苯丙氨酸寡肽的自组装:寡肽链长度的作用
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
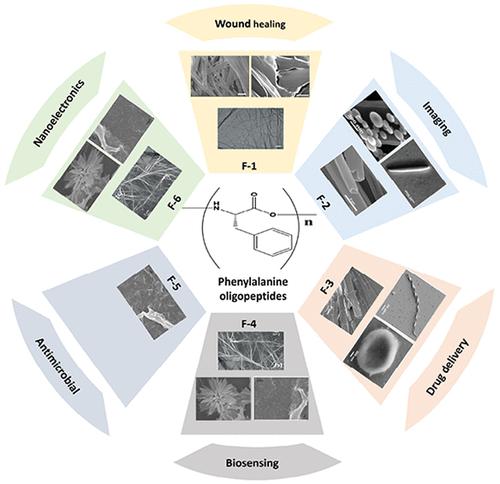
基于苯丙氨酸(F)的多肽的自组装是一个关键的研究领域,对先进生物材料和技术的发展具有潜在的意义。先前的研究表明,含有F-X残基(X = 1 ~ 6)的同源寡肽可以自组装成各种纳米微观结构,但寡肽链长度在这一过程中的作用尚不清楚。本文研究了F-X链长度对自组装过程和形态的影响,考虑了孵育条件和N端和/或C端capping基团的影响。形态,如原纤维或管状,是F-X寡肽自组装的典型结构,特别是具有偶数残基的结构。当F-X寡肽的一个或两个末端被封顶时,形成这种结构的倾向就会改变。高芳香族F-X寡肽表现出广泛的形态,这是由于堆叠的芳香族基团产生疏水核,导致缓慢形成缺乏明确形态的不良聚集体。末端电荷和寡肽主链上的旋盖基团影响自组装F-Xs (X >;1)通过驱动F-X单体之间的平行或反平行β链缔合。我们得出结论,寡肽链长度在f基肽的自组装过程中起着关键作用,短链可能导致形成更稳定和有序的结构。除链长外,还有其他因素影响其结构,包括溶剂类型、共溶剂性质(极性和挥发性)、寡肽浓度和温度。研究自组装过程的一个重大挑战是,由于F-X寡肽之间的溶解度变化,缺乏在相同孵育条件下促进自组装的溶剂。因此,需要额外的实验和数学研究来检查F-X寡肽在相同的孵育条件下(溶剂类型、助溶剂、肽浓度、pH和温度)的自组装,以生产可行的f基材料,并在先进的生物材料和技术中有潜在的应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Self-Assembly of Homo Phenylalanine Oligopeptides: Role of Oligopeptide Chain Length
The self-assembly of phenylalanine (F)-based peptides is a critical area of research with potential implications for the development of advanced biomaterials and technologies. Previous studies indicate that homo-oligopeptides with F-X residues (X = 1 to 6) can self-assemble into diverse nano-microstructures, but the role of oligopeptide chain length on this process remains unclear. This review investigates the role of F-X chain length on self-assembly processes and morphologies, considering the effect of incubation conditions and the capping group at the N and/or C terminals. Morphologies, such as fibrils or tubes, are typical structures that result from the self-assembly of F-X oligopeptides, especially, with an even number of residues. When one or two termini of the F-X oligopeptide are capped, the tendency to form such structures is altered. Highly aromatic F-X oligopeptides display a wide range of morphologies due to hydrophobic cores created by stacked aromatic groups leading to slow formation of poor aggregates without well-defined morphologies. The terminal charges and capping groups on the oligopeptide backbone affect the atomic-level structure of self-assembled F-Xs (X > 1) by driving parallel or antiparallel β-strand associations between F-X monomers. We conclude that oligopeptide chain length plays a critical role in the self-assembly process of F-based peptides and that shorter chains may lead to the formation of more stable and ordered structures. Besides chain length, several other factors influence the structures, including solvent type, cosolvent properties (polarity and volatility), oligopeptide concentrations, and temperature. A significant challenge in investigating self-assembly processes is the lack of a solvent that promotes self-assembly under identical incubation conditions due to solubility variations among F-X oligopeptides. Consequently, additional experimental and mathematical studies are required to examine the self-assembly of F-X oligopeptides under the same incubation conditions (solvent type, cosolvent, peptide concentration, pH, and temperature) to produce viable F-based materials with potential applications in advanced biomaterials and technologies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


