β -d-葡萄糖和Adonite添加剂增强al -空气电池性能:电化学和理论的结合研究
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
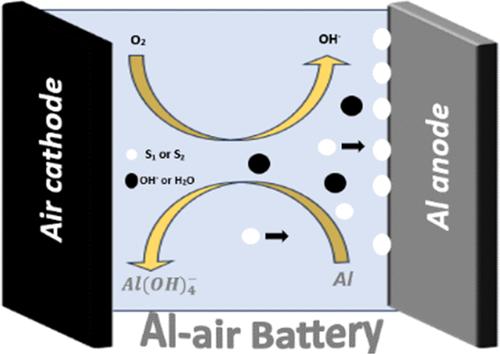
铝空气电池具有较高的理论能量密度,但其广泛应用受到析氢腐蚀的阻碍。本研究的重点是β (+) d-葡萄糖(S1)和Adonite (S2)作为Al-5052合金在4 M NaOH溶液中的潜在缓蚀剂。利用电化学技术、析氢评估和表面分析,我们的研究结果表明,S1和S2的阳极利用率分别提高了21.9%和21.1%。在10-3 m浓度下,S1和S2的抑制效率分别达到65.5%和65.1%。此外,S1和S2的引入显著提高了电池的标称比容量(S1为654 mA h g-1, S2为629 mA h g-1)和能量密度(S1为1922 W h kg-1, S2为1849 W h kg-1)。这些结果表明,使用这些添加剂管理电解质成分可以显着提高电池在碱性环境中的性能。密度泛函理论(DFT)和分子动力学(MD)分析支持了我们的实验结果,证实了阳极钝化和有益的分子相互作用的改善,有助于减少Al-5052合金的腐蚀。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Enhancing Al–Air Battery Performance with Beta-d-Glucose and Adonite Additives: A Combined Electrochemical and Theoretical Study
Al–air batteries are distinguished by their high theoretical energy density, yet their broader application is hindered by hydrogen evolution corrosion. This research focuses Beta (+) d-glucose (S1) and Adonite (S2) as potential corrosion inhibitors for the Al-5052 alloy within a 4 M NaOH solution. Utilizing electrochemical techniques, hydrogen evolution assessments, and surface analyses, our findings indicate enhancements in anode utilization by 21.9% for S1 and 21.1% for S2. Inhibition efficiency reached 65.5% for S1 and 65.1% for both additives at a concentration of 10–3 M. Additionally, the introduction of S1 and S2 markedly increased the nominal specific capacity (654 mA h g–1 for S1 and 629 mA h g–1 for S2) and energy density (1922 W h kg–1 for S1 and 1849 W h kg–1 for S2) of the batteries. These results suggest that managing the electrolyte composition with these additives can significantly enhance battery performance in alkaline environments. Supporting our experimental findings, density functional theory (DFT) and molecular dynamics (MD) analyses confirmed improved anode passivation and beneficial molecular interactions, contributing to the reduction of corrosion in the Al-5052 alloy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


