解锁天然沥青的亲核功能化潜力:接枝Pd(0) -二乙醇胺配合物作为升级联芳基合成的可回收催化剂
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
本研究介绍了一种天然沥青功能化的新方法,为将沥青质从道路升级为催化剂提供了新的机会。该工艺利用醋酸中的无金属声溴化技术将碳卤素取代基掺入天然沥青中。然后用二乙醇胺亲核取代这些位点,然后用Pd(0)络合,形成一种独特的接枝到天然沥青上的钯配合物。这种稳定的配合物在铃木反应中充当多相可回收催化剂。该配合物以聚乙二醇-400为绿色溶剂,在温和条件下促进芳基硼酸与各种邻位、间位和对取代芳基卤化物的反应。卤化物的离去基能力和取代基对两种反应物的电子和空间效应对反应转化率有显著影响。这种环境友好的工艺提供了广泛的底物范围(24个例子),并实现了联苯衍生物的优异产量。值得注意的是,它采用了自然衍生的催化支持,强调了其可持续性。这项研究有可能解开亲核试剂与天然沥青的键合,从而从这种可再生资源中开发新的功能材料。本文章由计算机程序翻译,如有差异,请以英文原文为准。
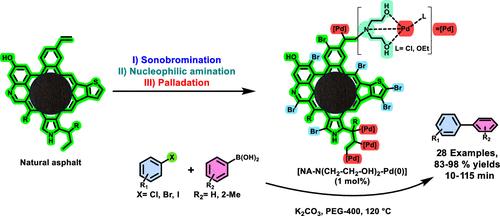
Unlocking the Nucleophilic Functionalization Potential of a Natural Asphalt: Grafting a Pd(0)–Diethanolamine Complex as a Recyclable Catalyst for Upgrading Biaryl Synthesis
This study introduces a novel method for functionalizing natural asphalt, presenting new opportunities for upgrading asphaltenes from road to a catalyst. The process utilizes a metal-free sonobromination technique in acetic acid to incorporate carbon–halogen substituents onto natural asphalt. These sites are then targeted by nucleophilic substitution with diethanolamine, followed by complexation with Pd(0) to create a unique palladium complex grafted onto natural asphalt. This stabilized complex serves as a heterogeneous and recoverable catalyst in the Suzuki reaction. This complex facilitates the reaction between aryl boronic acids and various ortho-, meta-, and para-substituted aryl halides under mild conditions using polyethylene glycol-400 as the green solvent. The reaction conversion rate is significantly influenced by the leaving group ability of the halides and the electronic and steric effects of the substituents on both reactants. This environmentally friendly process offers a broad substrate scope (24 examples) and achieves excellent yields of biphenyl derivatives. Notably, it employs a naturally derived catalytic support, underscoring its sustainability. This research potentially unlocks the bonding of nucleophiles to the natural asphalt for developing novel functional materials from this renewable resource.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


