基于27维势能面环聚合物分子动力学确定11原子反应速率系数:反ch3choo与H2O的反应
IF 4.6
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
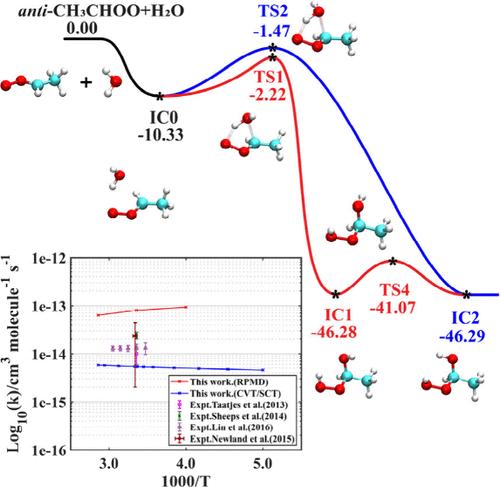
克里基中间体(CIs)是对流层中潜在的重要氧化剂和OH自由基的主要来源。抗ch3choo中间体已被证实是大气环境中CIs的重要组成部分。虽然以前的研究已经提供了一些实验和理论速率常数,但这些数据之间仍然存在不一致性,并且实验数据没有涵盖对流层中存在的全部温度范围。在此,我们利用基本不变神经网络方法建立了反ch3choo + H2O反应的精确全维(27维)势能面(PES),并在此基础上对涉及11个原子的复杂多通道反应进行了环聚合物分子动力学(RPMD)计算,这是目前计算限制的一个重大挑战。在250和350 K之间的RPMD速率系数比基于变分过渡态理论的结果大1个数量级。这种差异突出了两个氢转移通道之间明显的动力学效应和适度的量子效应。这项工作为标题反应提供了可靠的速率系数,这对于评估反ch3choo的大气命运和建立可靠的大气模型至关重要。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Determining Rate Coefficients for the 11-Atom Reaction via Ring Polymer Molecular Dynamics Based on a 27-Dimensional Potential Energy Surface: The Reaction between anti-CH3CHOO and H2O
Criegee intermediates (CIs) are potentially significant oxidants and a major source of OH radicals in the troposphere. The anti-CH3CHOO intermediate has been confirmed as a crucial component of CIs in the atmospheric environment. Although previous studies have provided some experimental and theoretical rate constants, inconsistencies among these data remain, and the experimental data do not cover the full range of temperatures present in the troposphere. Here, we developed an accurate full-dimensional (27-dimensional) potential energy surface (PES) for the anti-CH3CHOO + H2O reaction using the fundamental invariant-neural network approach and performed the ring polymer molecular dynamics (RPMD) calculations on the basis of this PES for this complex multichannel reaction involving 11 atoms, posing a significant challenge due to current computational limits. The RPMD rate coefficients between 250 and 350 K are ∼1 order of magnitude larger than the results based on variational transition-state theory. This discrepancy highlights pronounced dynamical effects and moderate quantum effects across the two hydrogen-transfer channels. This work provides reliable rate coefficients for the title reaction, which are vital for evaluating the atmospheric fate of anti-CH3CHOO and for developing reliable atmospheric models.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry Letters
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
9.60
自引率
7.00%
发文量
1519
审稿时长
1.6 months
期刊介绍:
The Journal of Physical Chemistry (JPC) Letters is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, chemical physicists, physicists, material scientists, and engineers. An important criterion for acceptance is that the paper reports a significant scientific advance and/or physical insight such that rapid publication is essential. Two issues of JPC Letters are published each month.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


