Mn(II)-卟啉酸亚硝基配合物光诱导的硝基阴离子/HNO释放
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
具有{MnNO}6构型的mni -亚硝基通常被认为具有低自旋的电子特性[mnni -NO+],因此从mni -亚硝基中释放HNO/ NO¯是不利的。虽然只有少数{MnNO}6已知在特定条件下释放NO,但迄今为止,中四基(4-甲氧基苯基)卟啉框架中的mnni -亚硝基是唯一在中性或阴离子第六配体存在下释放NO的报道。合成了Mn(II)-卟啉酸亚硝基配合物,1,[Mn(TTMPP2-)(NO)] {H2TTMPP = 5,10,15,20-四(3,4,5-三甲氧基苯基)卟啉}。发现配合物1在-40℃的二氯甲烷溶液中,在可见光存在下,向[FeIII(TPP)Cl]和[FeIII(dtc)3] {H2TPP =四苯基卟啉和dtc =二乙基二硫代氨基甲酸酯}提供硝基阴离子(NO-),生成相应的Fe(II)-亚硝基。进一步,当配合物1与等量的HBF4在可见光下搅拌时,观察到顶空气体中存在N2O。这反过来表明,在可见光下,配合物1在H+存在的情况下提供HNO。这些研究表明,在可见光存在下,配合物1充当NO-/HNO供体。本文章由计算机程序翻译,如有差异,请以英文原文为准。
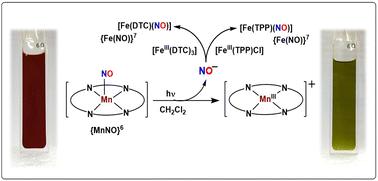
Photo-induced nitroxyl anion/HNO release from a nitrosyl complex of Mn(ii)–porphyrinate†
A nitrosyl complex of MnII–porphyrinate, has been synthesized and characterized. It was found to donate a nitroxyl anion (NO−) to suitable acceptors in dichloromethane solution in the presence of visible light. The evolution of N2O and the characteristic reaction with PPh3 in the presence of H+ confirms the NO−/HNO donation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


