利用WAAFLE分析人类微生物组中的横向基因转移事件
IF 20.5
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
摘要
横向基因转移(LGT),也称为水平基因转移,促进了微生物种群的基因组多样化。虽然之前的工作已经调查了与人类相关的微生物分离基因组中的LGT,但由于没有广泛采用的方法来表征复杂群落中的LGT,因此对个人微生物组中LGT的研究还不是很清楚。在这里,我们开发,基准测试和验证了一个计算算法(WAAFLE或工作流来注释汇编和查找LGT事件),以从组装的宏基因组中分析LGT。WAAFLE优先考虑特异性,同时保持对属间LGT的高灵敏度。将WAAFLE应用于来自不同身体部位的2,000个人类宏基因组,我们确定了100,000个高可信度的先前未表征的LGT(每个微生物基因组当量约2个)。这些富集了可移动元素,以及与破坏外源DNA相关的限制性修饰功能。LGT频率受生物地理、涉及对的系统发育相似性(如牙周梭菌和具核梭菌)和供体丰度的影响。这些力量表现为枢纽分类群与系统发育邻居的捐赠不平等的网络。我们的研究结果表明,人类微生物组LGT可能比以前描述的更普遍。本文章由计算机程序翻译,如有差异,请以英文原文为准。
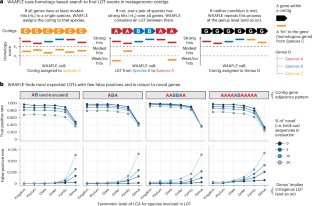

Profiling lateral gene transfer events in the human microbiome using WAAFLE
Lateral gene transfer (LGT), also known as horizontal gene transfer, facilitates genomic diversification in microbial populations. While previous work has surveyed LGT in human-associated microbial isolate genomes, the landscape of LGT arising in personal microbiomes is not well understood, as there are no widely adopted methods to characterize LGT from complex communities. Here we developed, benchmarked and validated a computational algorithm (WAAFLE or Workflow to Annotate Assemblies and Find LGT Events) to profile LGT from assembled metagenomes. WAAFLE prioritizes specificity while maintaining high sensitivity for intergenus LGT. Applying WAAFLE to >2,000 human metagenomes from diverse body sites, we identified >100,000 high-confidence previously uncharacterized LGT (~2 per microbial genome-equivalent). These were enriched for mobile elements, as well as restriction–modification functions associated with the destruction of foreign DNA. LGT frequency was influenced by biogeography, phylogenetic similarity of involved pairs (for example, Fusobacterium periodonticum and F. nucleatum) and donor abundance. These forces manifest as networks in which hub taxa donate unequally with phylogenetic neighbours. Our findings suggest that human microbiome LGT may be more ubiquitous than previously described. Applying WAAFLE to metagenomic data from human samples reveals over 100,000 putative LGTs not currently found in reference genomes, highlighting an underappreciated microbiome phenomenon.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


