天然产物白蜡林衍生物抗头颈部鳞状细胞癌的合成及药理评价
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
头颈部鳞状细胞癌(HNSCC)是最常见的恶性肿瘤之一,但临床药物治疗有限。通过天然产物文库的表型筛选,鉴定天然产物二黄芪多糖为抑制HNSCC细胞增殖的先导化合物。然而,由于生物活性较弱和代谢稳定性较差,制约了二苯丁醇作为抗鳞状细胞癌药物的进一步发展。在此,我们设计并合成了两个系列的新的二黄芪衍生物,通过引入各种吡喃糖环或亲水性基团来阻断易代谢的C-4位点,以提高抗肿瘤活性和药物样性质。其中,化合物A3对HNSCC细胞的抑制作用最强,IC50值为4.37 ~ 77.81 nM,对正常细胞的细胞毒性较弱(IC50 >;10μM)。机制上,它能有效抑制细胞增殖和迁移,诱导细胞周期阻滞和凋亡,并呈浓度依赖性。此外,A3具有显著改善的药代动力学特性,包括10倍以上的血浆暴露(AUC0-t: 541 vs 43.6 h*ng/mL),更好的口服生物利用度(F: 20.85% vs 2.70%),更低的全身血浆清除率(CL:1897 vs 24523 mL/h/kg),以及更长的半衰期(T1/2: 0.530 vs 0.108 h)。在肿瘤细胞异种移植模型中,A3能显著抑制CAL27肿瘤生长,TGI为42.2%,且无明显安全性问题,优于二苯三酚(TGI = 23.3%),提示其治疗HNSCC的潜力巨大。本文章由计算机程序翻译,如有差异,请以英文原文为准。

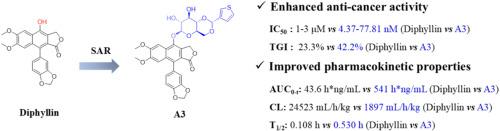
Synthesis and pharmacological evaluation of natural product diphyllin derivatives against head and neck squamous cell carcinoma
Head and neck squamous cell carcinoma (HNSCC) is one of the most common malignant tumors, but clinical drug treatments are limited. The natural product diphyllin was identified as a lead compound suppressing the proliferation of HNSCC cells through phenotypic screening of natural product library. However, further developments of diphyllin as an anti-HNSCC agent were restricted by the weak bioactivity and poor metabolic stability. Herein, we designed and synthesized two series of novel diphyllin derivatives that were achieved by introducing various pyranose rings or hydrophilic groups to block the easily metabolic C-4 site with the aim to improve antitumor activity and drug-like properties. Among these compounds, compound A3 showed the most potent inhibitory effects against HNSCC cells with IC50 values ranging from 4.37 to 77.81 nM and much less potent cytotoxicity against normal cells (IC50 > 10 μM). Mechanistically, it effectively inhibited cell proliferation and migration and induced the cell cycle arrest and apoptosis in a concentration-dependent manner. Besides, A3 possessed greatly improved pharmacokinetic properties including over 10-fold higher plasma exposure (AUC0-t: 541 vs 43.6 h∗ng/mL) and better oral bioavailability (F: 20.85 % vs 2.70 %), lower systemic plasma clearance (CL:1897 vs 24523 mL/h/kg), as well as longer half-life (T1/2: 0.530 vs 0.108 h) when compared to diphyllin. In a tumor cell xenograft model, A3 significantly suppressed the CAL27 tumor growth with a TGI of 42.2 % without obvious safety concern, which is superior to that of diphyllin (TGI = 23.3 %), suggesting great potential for treatment of HNSCC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


