核酸酶MRE11新抑制剂的发现
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
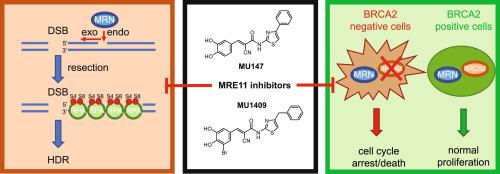
MRE11核酸酶是信号传导和处理DNA损伤以及解决停滞复制分叉的核心参与者。在这里,我们描述了新的MRE11抑制剂MU147和MU1409的鉴定和表征。这两种化合物对MRE11核酸酶的抑制比相对弱的最先进的抑制剂mirin更特异性和有效。它们还消除了依赖于MRE11核酸酶活性的双链断裂修复机制,而不损害ATM的激活。抑制MRE11也会损害停滞复制叉的新生链降解,并选择性地影响brca2缺陷细胞。本研究表明,我们新发现的化合物MU147和MU1409可以作为化学探针进一步探索MRE11的生物学作用,并支持药物抑制该核酸酶的潜在临床意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Discovery of new inhibitors of nuclease MRE11
MRE11 nuclease is a central player in signaling and processing DNA damage, and in resolving stalled replication forks. Here, we describe the identification and characterization of new MRE11 inhibitors MU147 and MU1409. Both compounds inhibit MRE11 nuclease more specifically and effectively than the relatively weak state-of-the-art inhibitor mirin. They also abrogate double-strand break repair mechanisms that rely on MRE11 nuclease activity, without impairing ATM activation. Inhibition of MRE11 also impairs nascent strand degradation of stalled replication forks and selectively affects BRCA2-deficient cells. Herein, we illustrate that our newly discovered compounds MU147 and MU1409 can be used as chemical probes to further explore the biological role of MRE11 and support the potential clinical relevance of pharmacological inhibition of this nuclease.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


