水在水泥水化产物和伴生矿物表面的分子结构和动力学:纳米级润湿性行为
IF 6.9
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
在沿海环境中,润湿性对水泥基材料的性能至关重要,在沿海环境中,盐水会加速材料的劣化。在分子水平上了解关键水化产物的润湿行为对于提高材料耐久性和开发有效的修复方案至关重要。本研究考察了水泥基材料中四种主要基质的润湿性:钙矾石(AFt)、硬石膏(CaSO4)、碳酸钙(CaCO3)和水合硅酸钙(C-S-H),利用分子动力学模拟分析了接触角、偶极子取向、界面键和能量相互作用。结果表明,基质特异性润湿性,AFt的接触角为8.04°,CaSO4为10.75°,CaCO3为15.61°,C-S-H为10.06°。值得注意的是,由于AFt和C-S-H与羟基和硅酸盐基团之间存在强大的氢键,因此可以使水渗透更深,从而提高润湿性。相比之下,在离子相互作用的驱动下,CaSO4和CaCO3表现出有限的水分子扩散,CaCO3表现出强烈的水吸附,限制了液滴的流动性。H2O分子不同的氢键和偶极子角分布说明了在不同基质上不同的润湿行为。这些发现促进了对润湿机制的分子水平理解,为设计用于沿海应用的耐用胶凝材料提供了有价值的见解,特别是在耐硫酸盐、快速硬化的海洋修复场景中。本文章由计算机程序翻译,如有差异,请以英文原文为准。
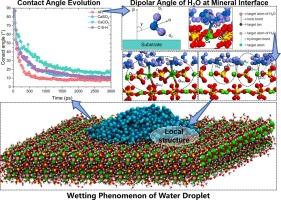
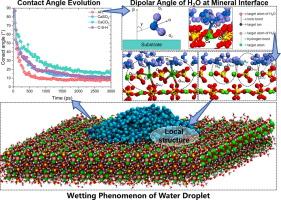
Molecular structure and dynamics of water on the surfaces of cement hydration products and associated Minerals: Nanoscale wettability behavior
Wettability is crucial to the performance of cement-based materials in coastal environments, where saline exposure accelerates deterioration. Understanding the wetting behavior of key hydration products at the molecular level is essential for enhancing material durability and developing effective repair solutions. This study examines the wettability of four principal substrates in cement-based materials: ettringite (AFt), anhydrite (CaSO4), calcium carbonate (CaCO3), and calcium silicate hydrate (C-S-H), utilizing molecular dynamics simulations to analyze contact angles, dipole orientations, interfacial bonding, and energy interactions. Results indicate substrate-specific wettability, with contact angles of 8.04° for AFt, 10.75° for CaSO4, 15.61° for CaCO3, and 10.06° for C-S-H. Notably, AFt and C-S-H allow deeper water penetration due to robust hydrogen bonding with hydroxyl and silicate groups, facilitating high wettability. In contrast, CaSO4 and CaCO3 display limited water molecule diffusion, driven by ionic interactions, with CaCO3 exhibiting strong water adsorption that constrains droplet mobility. The distinct hydrogen bond and dipole angle distributions of H2O molecules elucidate the varied wetting behaviors on each substrate. These findings advance the molecular-level understanding of wetting mechanisms, offering valuable insights for designing durable cementitious materials for coastal applications, especially in sulfate-resistant, rapid-hardening marine repair scenarios.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


