VOX/Al2O3催化丙烷氧化脱氢:催化剂表征及失活行为研究
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
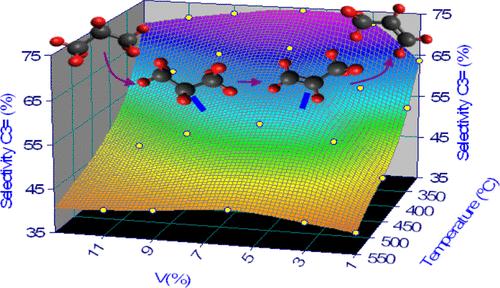
碳氢化合物的氧化脱氢(ODH)将丙烷和/或乙烷转化为丙烯和/或乙烯,这是化学工业中重要的化合物。与蒸汽裂解不同,ODH提供了较高的烯烃理论转化率和较低的能耗。采用湿浸渍法制备了VOX/Al2O3催化剂,研究了丙烷制丙烯的ODH反应。反应温度为300 ~ 550℃,C3/O2比为1.6 ~ 3.3,钒含量为1 ~ 11wt %。结果表明,这些参数是影响催化剂在ODH中性能的关键实验变量。由于单体和聚合VOX的存在,中间钒负载的催化剂表现出最佳的酸性位点数量,导致对丙烯的高选择性。程序升温氧化分析表明,焦炭沉积与VOX聚合程度成正比。本文章由计算机程序翻译,如有差异,请以英文原文为准。
VOX/Al2O3 Catalyzed Oxidative Dehydrogenation of Propane: Focus on Catalyst Characterization and Deactivation Behavior
Oxidative dehydrogenation of hydrocarbons (ODH) converts propane and/or ethane into propylene and/or ethylene, which are important compounds in the chemical industry. Unlike steam cracking, ODH offers a high theoretical conversion to olefins and lower energy consumption. The ODH reaction of propane to propylene was studied using VOX/Al2O3 catalysts prepared by wet impregnation. The reaction conditions were widely varied, with temperatures ranging from 300 to 550 °C, the C3/O2 ratio from 1.6 to 3.3, and the vanadium content from 1 to 11 wt %. It was observed that these parameters are key experimental variables that influence the performance of the catalysts in ODH. Catalysts with intermediate vanadium loading exhibit an optimal amount of acidic sites due to the presence of monomeric and polymeric VOX species, resulting in high selectivity toward propylene. Temperature-programmed oxidation analyses showed that coke deposition is proportional to the degree of VOX polymerization.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


