基于结构的β -极光激酶双抑制剂治疗癌症的合理设计与评价
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
同时抑制溴域、外端结构域和极光激酶是一种很有前途的抗癌治疗策略。基于我们之前对β -激酶双抑制剂的研究,我们采用分子对接的方法设计了新的β -极光激酶A双抑制剂。通过多轮优化,在BRD4与抑制剂27结合的已解共晶结构的指导下,我们最终获得了一系列高效的双BET-Aurora kinase a抑制剂。化合物38对BRD4和Aurora kinase a均具有较强的亲和力,对多种肿瘤细胞系均有良好的抗增殖活性,具有良好的药代动力学特征,在肾细胞癌和结肠癌异种移植模型中具有良好的抗肿瘤效果,TGI分别为45.99%和53.06%。化合物38的开发强化了合理设计可以实现针对特定激酶和溴结构域蛋白的双重抑制剂的概念。本文章由计算机程序翻译,如有差异,请以英文原文为准。
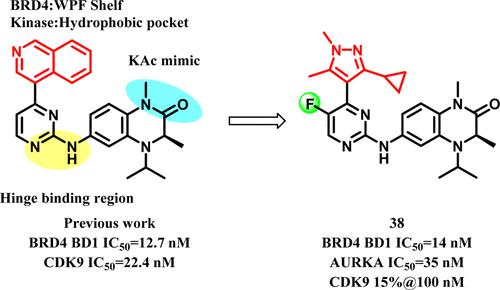
Structure-Based Rational Design and Evaluation of BET-Aurora Kinase Dual-Inhibitors for Treatment of Cancers
Simultaneous inhibition of the bromodomain and extra-terminal domain and Aurora kinases is a promising anticancer therapeutic strategy. Based on our previous study on BET-kinase dual inhibitors, we employed the molecular docking approach to design novel dual BET-Aurora kinase A inhibitors. Through several rounds of optimization and with the guidance of the solved cocrystal structure of BRD4 bound to inhibitor 27, we finally obtained a series of highly potent dual BET-Aurora kinase A inhibitors. Compound 38 exhibited strong affinity toward both BRD4 and Aurora kinase A. It also showed good antiproliferative activities on diverse cancer cell lines, good pharmacokinetic profiles, and favorable antitumor efficacy in renal cell cancer and colon cancer xenograft models with TGI of 45.99% and 53.06%, respectively. The development of compound 38 reinforces the concept that a rational design may achieve dual inhibitors targeting specific kinases and bromodomain proteins.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


