力纳米显微镜显示金黄色葡萄球菌铁调控的表面决定蛋白B和宿主toll样受体4之间的应力激活粘附
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
金黄色葡萄球菌铁调控的表面决定蛋白B (IsdB)最近被证明与toll样受体4 (TLR4)结合,从而在先天免疫细胞中诱导强烈的炎症反应。目前尚未解决的两个问题是:(1)IsdB-TLR4相互作用的分子机制是什么?(ii)它是否也在非免疫系统中发挥作用?在这里,我们使用单分子实验证明了IsdB结合TLR4的弱力和极强的力,并且在105 pN/s的加载速率下,物理应力可维持高达2000 pN的力,从而显著增加了分子复合物的机械稳定性。我们还发现,TLR4与IsdB的结合介导了时间依赖性细菌对内皮细胞的粘附,这表明这种结合在细胞侵袭中的作用。我们的研究结果指出IsdB在病原体-宿主相互作用中的功能,即在流体剪切应力下介导细菌对宿主内皮细胞的强粘附,迄今为止尚不清楚。在纳米医学中,这种应力依赖性粘附代表了针对金黄色葡萄球菌耐药菌株的创新治疗的潜在靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
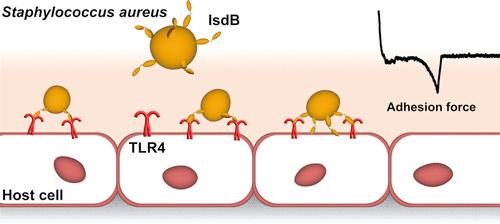
Force Nanoscopy Demonstrates Stress-Activated Adhesion between Staphylococcus aureus Iron-Regulated Surface Determinant Protein B and Host Toll-like Receptor 4
The Staphylococcus aureus iron-regulated surface determinant protein B (IsdB) has recently been shown to bind to toll-like receptor 4 (TLR4), thereby inducing a strong inflammatory response in innate immune cells. Currently, two unsolved questions are (i) What is the molecular mechanism of the IsdB-TLR4 interaction? and (ii) Does it also play a role in nonimmune systems? Here, we use single-molecule experiments to demonstrate that IsdB binds TLR4 with both weak and extremely strong forces and that the mechanostability of the molecular complex is dramatically increased by physical stress, sustaining forces up to 2000 pN, at a loading rate of 105 pN/s. We also show that TLR4 binding by IsdB mediates time-dependent bacterial adhesion to endothelial cells, pointing to the role of this bond in cell invasion. Our findings point to a function for IsdB in pathogen–host interactions, that is, mediating strong bacterial adhesion to host endothelial cells under fluid shear stress, unknown until now. In nanomedicine, this stress-dependent adhesion represents a potential target for innovative therapeutics against S. aureus-resistant strains.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


