新型木质素非离子表面活性剂的绿色合成
IF 7.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
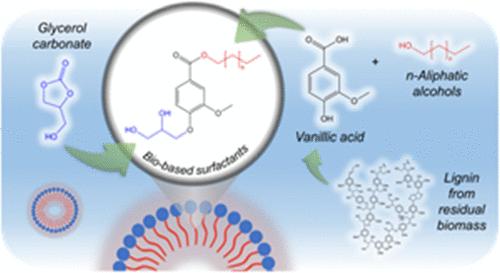
表面活性剂在日常生活和工业应用中都发挥着至关重要的作用。遗憾的是,大多数表面活性剂仍然来自化石资源,而且不能生物降解,因此需要无毒的生物替代品。木质素是木质纤维素生物质中的一种生物聚合物,是富含酚类分子的生物芳香化合物的可再生来源,因此适合进一步改性。然而,其对化学和生化过程的不适应性阻碍了其向高附加值产品的转化。在这项工作中,我们开发了一种在温和条件下制备新型生物基表面活性剂的可持续合成工艺。起始材料是香草酸(VA),它是一种木质素衍生的模型化合物。通过利用几种不同链长(C4-C10)的伯族脂肪醇来制备香草酸酯,从而获得非极性尾部,在 120 °C 下 45 分钟后实现完全转化。极性头是在纯净条件下与碳酸甘油酯直接偶联合成的,取代了有毒的环氧氯丙烷,提高了产率(63.1% 对 20.5%)。利用芘荧光法对所得产品进行了表征,以证明其表面活性剂特性并测量其临界胶束浓度(CMC),结果表明非极性尾的长度与 CMC 之间存在相关性。C8 衍生物显示出最低的 CMC,估计值为 5.05 × 10-5 M。这项工作证明了将木质素衍生平台化学品转化为可再生非离子表面活性剂的潜力,其应用范围十分广泛。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Green Synthesis of Cutting-Edge Nonionic Surfactants Derived from Lignin
Surfactants play a crucial role in both everyday life and industrial applications. Unfortunately, most are still derived from fossil sources and are not biodegradable, highlighting the need for nontoxic, biologically derived alternatives. Lignin is a biopolymer found in lignocellulosic biomass, which represents a renewable source of bioaromatic compounds rich in phenolic moieties, making it suitable for further modifications. However, its recalcitrance to chemical and biochemical processes hinders its conversion to value-added products. In this work, a sustainable synthetic process for the preparation of new biobased surfactants under mild conditions was developed. The starting material was vanillic acid (VA), which is a lignin-derived model compound. VA esters were prepared by exploiting several primary aliphatic alcohols with different chain lengths (C4–C10) to obtain the nonpolar tail, achieving a complete conversion after 45 min at 120 °C. The polar head was synthesized by direct coupling with glycerol carbonate in neat conditions, replacing the use of toxic epichlorohydrin, and improving yields (63.1% vs 20.5%). The resulting products were characterized by using the pyrene fluorescence method to prove their surfactant behavior and measure their critical micelle concentration (CMC), showing a correlation between the length of the nonpolar tail and the CMC. The C8 derivative showed the lowest CMC, with an estimated value of 5.05 × 10–5 M. This work demonstrates the potential for converting lignin-derived platform chemicals into renewable nonionic surfactants for a wide range of applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


