人锌结合半胱氨酸蛋白质组
IF 45.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
锌是一种必需的微量营养素,它调节了广泛的生理过程,最常见的是通过锌与蛋白质半胱氨酸残基的结合。尽管半胱氨酸对蛋白质功能的调节至关重要,但大多数人类蛋白质组中受锌结合影响的半胱氨酸位点仍未确定。在这里,我们开发了锌结合半胱氨酸蛋白组的深度定量图谱ZnCPT。我们定义了6173种锌结合半胱氨酸,揭示了生物学主要领域中受组成型或诱导型锌结合影响的蛋白质家族。ZnCPT能够系统地发现锌调节的结构,酶和变构功能域。在此基础上,我们确定了52种受锌结合的癌症遗传依赖性和对锌诱导的细胞毒性敏感的恶性肿瘤。我们发现锌调节谷胱甘肽还原酶(GSR)的机制,它驱动GSR依赖性肺癌的细胞死亡。我们提供ZnCPT作为了解锌调节蛋白质功能机制的资源。本文章由计算机程序翻译,如有差异,请以英文原文为准。
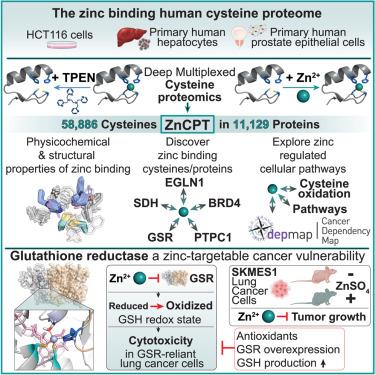
The human zinc-binding cysteine proteome
Zinc is an essential micronutrient that regulates a wide range of physiological processes, most often through zinc binding to protein cysteine residues. Despite being critical for modulation of protein function, the cysteine sites in the majority of the human proteome that are subject to zinc binding remain undefined. Here, we develop ZnCPT, a deep and quantitative mapping of the zinc-binding cysteine proteome. We define 6,173 zinc-binding cysteines, uncovering protein families across major domains of biology that are subject to constitutive or inducible zinc binding. ZnCPT enables systematic discovery of zinc-regulated structural, enzymatic, and allosteric functional domains. On this basis, we identify 52 cancer genetic dependencies subject to zinc binding and nominate malignancies sensitive to zinc-induced cytotoxicity. We discover a mechanism of zinc regulation over glutathione reductase (GSR), which drives cell death in GSR-dependent lung cancers. We provide ZnCPT as a resource for understanding mechanisms of zinc regulation of protein function.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


