氧化还原激活的过饱和铈固溶体作为动态催化剂实现低温乙苯氧化脱氢反应
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
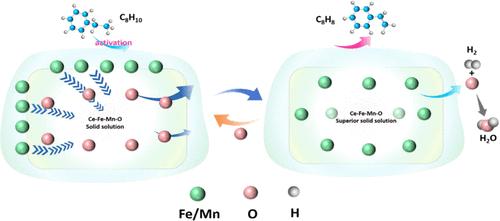
Redox-Activated Supersaturation of Ceria Solid Solution as a Dynamic Catalyst Enabling Low-Temperature Ethylbenzene Oxidative Dehydrogenation
Dynamic structural changes in the reactive environment often lead to catalyst deactivation in the thermal-catalysis field. Taking advantage of the dynamic changes in bulk phases, interfaces, and surface structures to design highly active catalysts is a unique but important strategy. Herein, we report a supersaturated ceria solid solution catalyst enabling a styrene yield of 91.8% over extended redox cycles at 430 °C in the redox oxidative dehydrogenation (ODH) of ethylbenzene. In-situ characterizations reveal that the oxygen anions (O2–) and transition-metal cations (Fe and Mn) reversibly shuttle through a ceria solid solution (bulk ↔ surface) in a K–Ce0.47Fe0.2Mn0.33O2−δ catalyst during the redox ODH process. The ceria solid solution acts as a dynamic transition-metal cations/oxygen reservoir, creating atomic interfaces of K–Fe–O/K–Mn–O and an oxygen gateway for efficient ethylbenzene ODH. The findings concerning the formation of a supersaturated ceria solid solution and cations, lattice oxygen migration, and the coupling between oxygen donation and catalytic reactions offer new strategies for designing high-performance dynamic catalysts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


