用芳基自由基测定法监测光活化钯催化芳基卤化物与烯的偶联过程中的自由基中间体
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
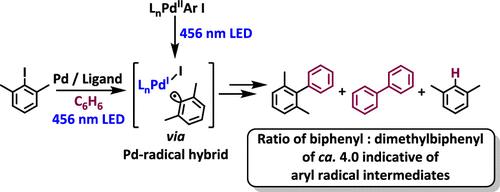
Monitoring Radical Intermediates in Photoactivated Palladium-Catalyzed Coupling of Aryl Halides to Arenes by an Aryl Radical Assay
An aryl radical assay is used to provide information about the formation of aryl radicals from aryl halides in coupling reactions to arenes in the presence of palladium sources and under LED irradiation (λ = 456 nm). The assay uses 2-halo-m-xylenes as substrates. Aryl radical formation is indicated both by a defined product composition and by signature deuterium isotope effects. Comparison with our recently published results for corresponding ground-state palladium-catalyzed reactions shows three principal differences: (i) in the photoactivated reactions, evidence supports the formation of aryl radical intermediates with all the phosphine ligands tested, in contrast to thermal ground-state chemistry where only specific ligands had encouraged this pathway, while others had promoted a nonradical coupling mechanism; (ii) oxidative addition complexes that are formed from the reaction of Pd(0) sources with aryl halides react under photoactivation to form biaryl coupled products through radical intermediates, in contrast to their behavior under thermal activation – so Ar–Pd bonds are homolyzed under LED irradiation; (iii) the photoreactions work well with mild bases like Cs2CO3, while the thermal reactions required KOtBu as the base due to the different roles for base under the thermal versus photochemical mechanisms.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


