丙烯酸氢胺化合成β-丙氨酸的天冬氨酸酶的生物信息学和计算支持重新设计
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
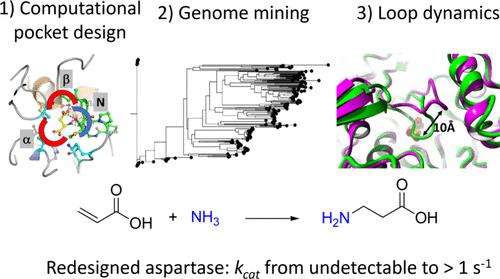
天冬氨酸氨解酶催化富马酸可逆胺化成l-天冬氨酸。最近的研究表明,来自芽孢杆菌sp. YM55-1 (AspB)的热稳定酶可以被工程用于取代β-氨基酸的对端选择性生产。该反应对丙烯酸转化为β-丙氨酸具有吸引力,而β-丙氨酸是制备生物活性化合物的重要组成部分。在这里,我们描述了一种生物信息学和计算方法,旨在介绍β-丙氨酸合成活性。采用了三种策略:首先,我们重新设计了AspB的α-羧酸结合袋,以引入与丙烯酸的活性。接下来,通过基因组挖掘鉴定不同的模板酶,配备重新设计的α-羧酸口袋,并研究β-丙氨酸合成,得到活性更好的变体。第三,利用能量计算对覆盖活性位点和含有催化丝氨酸的ss环的相互作用进行计算重新设计,以稳定反应构象,从而进一步提高所需的β-丙氨酸合成活性。得到了不同的改良酶,最佳的改良酶对丙烯酸的kcat值至少为0.6 ~ 1.5 s-1, KM值在高mM范围内。由于野生型酶的β-丙氨酸产量低于检测限,这表明kcat/Km至少提高了1000倍。重新设计的AspB 6倍突变体和来自caldisaponilyticus的类似工程天冬氨酸酶的晶体结构显示,它们的无配体结构具有封闭(活性)构象的ss环,而野生型AspB仅在底物结合酶中观察到这种结构。alphafold生成的模型表明,为丙烯酸氢胺化而重新设计的其他天冬氨酸酶变体也倾向于具有封闭构象的环的3D结构。结合结合袋重新设计、基因组挖掘和增强的活性位点环闭合,从而创建了有效的β-丙氨酸合成天冬氨酸的变体。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Bioinformatics and Computationally Supported Redesign of Aspartase for β-Alanine Synthesis by Acrylic Acid Hydroamination
Aspartate ammonia lyases catalyze the reversible amination of fumarate to l-aspartate. Recent studies demonstrate that the thermostable enzyme from Bacillus sp. YM55–1 (AspB) can be engineered for the enantioselective production of substituted β-amino acids. This reaction would be attractive for the conversion of acrylic acid to β-alanine, which is an important building block for the preparation of bioactive compounds. Here we describe a bioinformatics and computational approach aimed at introducing the β-alanine synthesis activity. Three strategies were used: First, we redesigned the α-carboxylate binding pocket of AspB to introduce activity with the acrylic acid. Next, different template enzymes were identified by genome mining, equipped with a redesigned α-carboxylate pocket, and investigated for β-alanine synthesis, which yielded variants with better activity. Third, interactions of the SS-loop that covers the active site and harbors a catalytic serine were computationally redesigned using energy calculations to stabilize reactive conformations and thereby further increase the desired β-alanine synthesis activity. Different improved enzymes were obtained and the best variants showed kcat values with acrylic acid of at least 0.6–1.5 s–1 with KM values in the high mM range. Since the β-alanine production of wild-type enzyme was below the detection limit, this suggests that the kcat/Km was improved by at least 1000-fold. Crystal structures of the 6-fold mutant of redesigned AspB and the similarly engineered aspartase from Caenibacillus caldisaponilyticus revealed that their ligand-free structures have the SS-loop in a closed (reactive) conformation, which for wild-type AspB is only observed in the substrate-bound enzyme. AlphaFold-generated models suggest that other aspartase variants redesigned for acrylic acid hydroamination also prefer a 3D structure with the loop in a closed conformation. The combination of binding pocket redesign, genome mining, and enhanced active-site loop closure thus created effective β-alanine synthesizing variants of aspartase.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


