细胞外囊泡对气孔的移植可向旁观者细胞传播嗜热症
IF 45.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
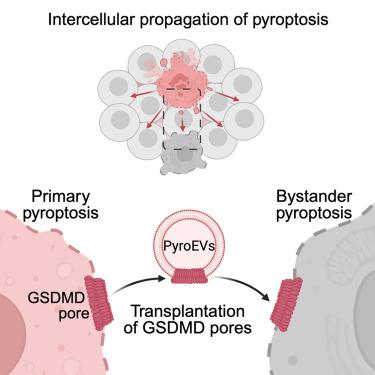
Transplantation of gasdermin pores by extracellular vesicles propagates pyroptosis to bystander cells
Pyroptosis mediated by gasdermins (GSDMs) plays crucial roles in infection and inflammation. Pyroptosis triggers the release of inflammatory molecules, including damage-associated molecular patterns (DAMPs). However, the consequences of pyroptosis—especially beyond interleukin (IL)-1 cytokines and DAMPs—that govern inflammation are poorly defined. Here, we show intercellular propagation of pyroptosis from dying cells to bystander cells in vitro and in vivo. We identified extracellular vesicles (EVs) released by pyroptotic cells as the propagator of lytic death to naive cells, promoting inflammation. DNA-PAINT super-resolution and immunoelectron microscopy revealed GSDMD pore structures on EVs released by pyroptotic cells. Importantly, pyroptotic EVs transplant GSDMD pores on the plasma membrane of bystander cells and kill them. Overall, we demonstrate that cell-to-cell vesicular transplantation of GSDMD pores disseminates pyroptosis, revealing a domino-like effect governing disease-associated bystander cell death.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


