TRACERx分析发现FAT1在通过Hippo信号调节染色体不稳定性和全基因组加倍中的作用
IF 17.3
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
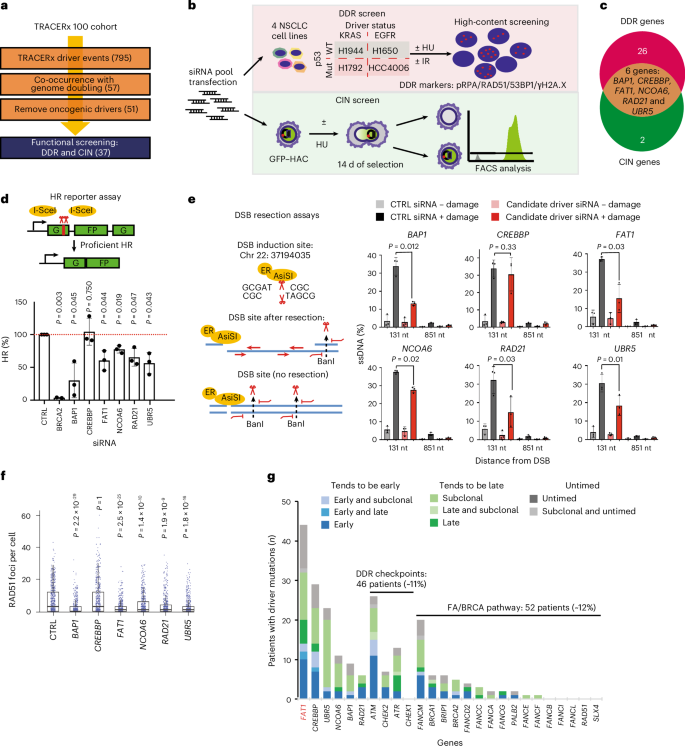
染色体不稳定性(CIN)在实体肿瘤中很常见,并通过增加肿瘤内异质性来促进进化适应和不良预后。对TRACERx非小细胞肺癌(NSCLC)队列中驱动事件的系统表征发现,包括FAT1在内的6个基因的遗传改变导致同源重组(HR)修复缺陷和CIN。使用正交遗传和实验方法,我们证明了FAT1改变在基因组加倍之前是正选择的,并且与HR缺乏有关。FAT1消融导致持续的复制应激,有丝分裂失败率升高,核变形和结构CIN升高,包括染色体易位和径向染色体。FAT1缺失通过YAP1的失调导致全基因组加倍(一种数字CIN)。YAP1的共耗尽部分挽救了FAT1缺失引起的数值CIN,但不能缓解HR缺陷,也不能缓解结构性CIN。重要的是,过表达组成活性YAP15SA足以诱导数值CIN。综上所述,我们发现NSCLC中FAT1的缺失通过两种不同的下游机制减弱HR并加剧CIN,从而导致肿瘤异质性增加。本文章由计算机程序翻译,如有差异,请以英文原文为准。

TRACERx analysis identifies a role for FAT1 in regulating chromosomal instability and whole-genome doubling via Hippo signalling
Chromosomal instability (CIN) is common in solid tumours and fuels evolutionary adaptation and poor prognosis by increasing intratumour heterogeneity. Systematic characterization of driver events in the TRACERx non-small-cell lung cancer (NSCLC) cohort identified that genetic alterations in six genes, including FAT1, result in homologous recombination (HR) repair deficiencies and CIN. Using orthogonal genetic and experimental approaches, we demonstrate that FAT1 alterations are positively selected before genome doubling and associated with HR deficiency. FAT1 ablation causes persistent replication stress, an elevated mitotic failure rate, nuclear deformation and elevated structural CIN, including chromosome translocations and radial chromosomes. FAT1 loss contributes to whole-genome doubling (a form of numerical CIN) through the dysregulation of YAP1. Co-depletion of YAP1 partially rescues numerical CIN caused by FAT1 loss but does not relieve HR deficiencies, nor structural CIN. Importantly, overexpression of constitutively active YAP15SA is sufficient to induce numerical CIN. Taken together, we show that FAT1 loss in NSCLC attenuates HR and exacerbates CIN through two distinct downstream mechanisms, leading to increased tumour heterogeneity. Lu et al. perform systematic functional analyses using data from the TRACERx cohort of patients with non-small-cell lung cancer and delineate how FAT1 regulates homologous recombination repair, chromosomal instability and whole-genome doubling with distinct mechanisms.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


