一锅法制备zncl2掺杂介孔二氧化硅纳米颗粒高效去除硝酸盐。
IF 7.7
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
硝酸盐是天然氮循环中的重要营养物。然而,人类活动使水生生态系统中的硝酸盐含量超过自然阈值,对人类健康和环境构成风险。本文采用一锅法合成了掺杂zncl2的介孔二氧化硅纳米颗粒(ZnCl2@MSN),简化了工艺流程,减少了时间和能耗。均相介孔ZnCl2@MSN主要通过离子交换机制(70.97%)和静电吸引(29.03%)对水中硝酸盐进行了高效去除。在合成水中(0.75 g ZnCl2@MSN;100ml 100mg L-1硝酸溶液,不调节pH值,室温(25℃)。间歇式吸附对实际水样的去除率可达99.05%±5.88%。实验数据最符合Langmuir等温线模型(R2 = 0.9966)和伪二阶模型(R2 = 0.9985)。热力学研究揭示了一个自发的放热吸附过程。硝酸盐对共存离子的耐受性为ZnCl2@MSN,顺序为PO43- > CO32- > F- > Cl- > SO42- > Br-,材料可重复使用3次。这项研究突出了ZnCl2@MSN的显著优势,与之前报道的类似材料相比,它通过更简单,更节能的程序合成。尽管其流线型制备,ZnCl2@MSN实现了优越的吸附能力,需要较少的吸附剂进行有效的处理。这不仅可以最大限度地减少废物产生,还可以降低运营成本。此外,其出色的可重用性提高了成本效率,使其成为一种非常实用的解决方案。重要的是,对处理过的水的评估证实,锌的浓度仍然远远低于EPA的排放限制。这些发现强调了ZnCl2@MSN作为一种先进、可持续和经济的硝酸盐去除材料的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
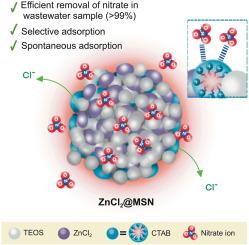
ZnCl2-doped mesoporous silica nanoparticles prepared via a simple one-pot method for highly efficient nitrate removal
Nitrate is a crucial nutrient in the natural nitrogen cycle. However, human activities have elevated nitrate levels in aquatic ecosystems beyond natural thresholds, posing risks to human health and the environment. In this work, ZnCl2-doped mesoporous silica nanoparticles (ZnCl2@MSN) were synthesized using a one-pot preparation method, leading to a streamlined process with reduced time and energy consumption. The homogeneous mesoporous ZnCl2@MSN exhibited efficient nitrate removal from water, primarily through ion exchange mechanisms (70.97%), with additional support from electrostatic attraction (29.03%). A removal efficiency of 85.11% ± 4.96% was achieved, with a maximum removal capacity of 22.37 mg g−1 within 15 min in synthetic water (0.75 g ZnCl2@MSN; 100 mL of 100 mg L−1 nitrate solution, without pH adjustment, at room temperature (25 °C)). Furthermore, batch adsorption demonstrated a removal efficiency of up to 99.05% ± 5.88% for real water samples. The experimental data fitted best to the Langmuir isotherm model (R2 = 0.9966) and the pseudo-second-order model (R2 = 0.9985). Thermodynamic studies revealed a spontaneous and exothermic adsorption process. Nitrate exhibited tolerance to coexisting ions using ZnCl2@MSN in the order of PO43− > CO32− > F− > Cl− > SO42− > Br−, and the material could be reused up to three times. This research highlights the significant advantages of ZnCl2@MSN, which is synthesized through a simpler and more energy-efficient procedure compared to similar materials reported previously. Despite its streamlined preparation, ZnCl2@MSN achieves a superior adsorption capacity, requiring less adsorbent for effective treatment. This not only minimizes waste generation but also reduces operational costs. Furthermore, its excellent reusability enhances cost-efficiency, making it a highly practical solution. Importantly, the evaluation of treated water confirmed that the zinc concentration remained well below the EPA discharge limit. These findings underscore the potential of ZnCl2@MSN as an advanced, sustainable, and economical material for nitrate removal.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Environmental Research
环境科学-公共卫生、环境卫生与职业卫生
CiteScore
12.60
自引率
8.40%
发文量
2480
审稿时长
4.7 months
期刊介绍:
The Environmental Research journal presents a broad range of interdisciplinary research, focused on addressing worldwide environmental concerns and featuring innovative findings. Our publication strives to explore relevant anthropogenic issues across various environmental sectors, showcasing practical applications in real-life settings.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


