基于溶液的Cu3Si生成复分解反应途径的探索
IF 7
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
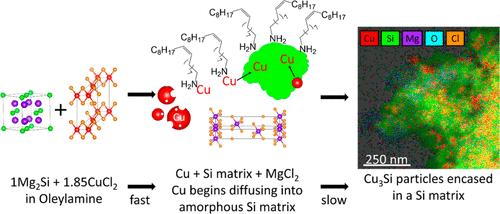
硅化铜Cu3Si具有广泛的应用,包括催化、光伏和储能。Cu-Si相图的复杂性使得合成一种具有控制化学计量和高纯度的相具有挑战性。本文所描述的特定Cu3Si相通常是用传统的固态方法制备的。研究表明,将胶体溶液法与复分解法相结合,可以成功合成Cu3Si@Si/SiO2基质颗粒。反应途径出奇地复杂和微妙。通过粉末x射线衍射、透射电子显微镜、扫描选择显微镜和能量色散x射线能谱(EDS),我们发现,与油胺(OLA)中的Mg2Si和CuCl2直接进入Cu3Si@Si/SiO2基体颗粒(正如你可能在固态中所期望的那样)不同,它是通过两步过程进行的。在第一步中,Mg2Si快速将Cu-OLA配合物还原为Cu0,生成MgCl2和不稳定的非晶态Si矩阵,这些矩阵被OLA覆盖。接下来,OLA帮助Cu穿梭到Si基体中,Cu扩散到不稳定的非晶结构中,形成嵌入在Si/SiO2基体中的Cu3Si颗粒。我们证明溶剂是控制该反应的关键。最后,利用扫描透射电子显微镜/能谱、电化学循环和x射线光电子能谱对Cu3Si颗粒包裹的基体进行了选择性分析。这表明基体中含有活性Si和微量Mg,且基体容易氧化,主要氧化为SiO2。这种独特的Cu3Si@Si/SiO2基质颗粒合成方法,虽然仍然限制了扩散,但结合了溶液和分解方法,降低了固态方法中常见的高地层能垒。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Exploring the Solution-Based Metathesis Reaction Pathway Toward Cu3Si Formation
Copper silicide, Cu3Si, has a wide range of applications, including catalysis, photovoltaics, and energy storage. The complexity of the Cu–Si phase diagram makes synthesizing one phase with control over stoichiometry and high purity challenging. The specific Cu3Si phase described herein is more typically made by using traditional solid-state methods. We demonstrate that Cu3Si@Si/SiO2 matrix particles can be successfully synthesized by combining colloidal solution and metathesis methods. The reaction pathway is surprisingly complex and nuanced. Using powder X-ray diffraction, transmission electron microscopy, scanning election microscopy, and energy dispersive X-ray spectroscopy (EDS), it was found that rather than Mg2Si and CuCl2 in oleylamine (OLA) proceeding directly to Cu3Si@Si/SiO2 matrix particles (as you might expect in the solid state), it proceeds through a two-step process. In the first step, Mg2Si quickly reduces the Cu–OLA complex to Cu0, resulting in MgCl2 and destabilized, amorphous Si matrices, which are capped with OLA. Next, OLA aids in shuttling Cu to the Si matrix, and Cu diffuses into the destabilized, amorphous structure to form Cu3Si particles embedded in a Si/SiO2 matrix. We show that the solvent is critical for controlling this reaction. Finally, the matrix encasing the Cu3Si particles was selectively analyzed by scanning transmission electron microscopy/EDS, electrochemical cycling, and X-ray photoelectron spectroscopy. This revealed that the matrix contains active Si with minimal amounts of Mg, and the matrix readily oxidizes, mainly to SiO2. This unique synthesis of Cu3Si@Si/SiO2 matrix particles, although still diffusion-limited, combines solution and metathesis methods to lower the high formation energy barrier commonly observed in solid-state methods.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


