氧化还原诱导的磷环双根素二聚化
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
尽管第一个例子在二十多年前就被分离出来了,但人们对稳定的以磷为中心的双根碱的氧化还原化学知之甚少。在这项研究中,我们研究了PPIPh,一种具有二磷茚基骨架的双根碱,并证明了它具有氧化和还原反应的能力。与二茂铁的单电子氧化产生一种指示笼状化合物,该化合物在结构上与二苯二烯(C10H10)的异构体有关。用一半当量的[Co2(CO)8]还原PPIPh会形成双金属配合物2,其中包含一个双(苯二膦孔)配体。本文章由计算机程序翻译,如有差异,请以英文原文为准。
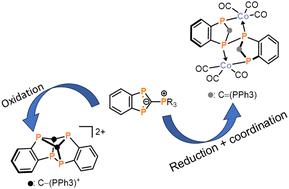
Redox-induced dimerisations of a phosphacyclic biradicaloid†
Despite the first examples being isolated more than two decades ago, little is known about the redox chemistry of stable phosphacyclic biradicaloids. Here, we demonstrate that a biradicaloid featuring a diphosphaindenyl backbone is able to undergo both oxidation and reduction reactions. One-electron oxidation results in the formation of a dicationic cage compound structurally related to an isomer of hypostrophene (C10H10). Reduction of with [Co2(CO)8] results in the formation of the bimetallic complex , which contains a bis(benzodiphosphole) ligand.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


