多组学方法揭示TGF-β信号驱动的牙周组织干细胞衰老
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
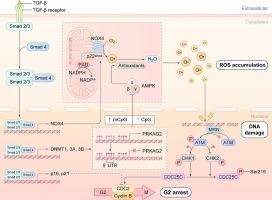
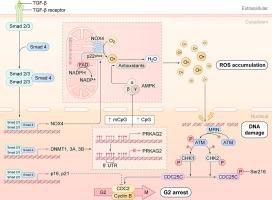
牙周韧带(PDL)是一种动态结缔组织,将牙齿固定在牙槽骨上,使牙齿保持并促进持续的更替。牙周组织的完整性由牙周韧带干细胞(PDLSCs)维持,其功能障碍和衰老会破坏组织稳态,阻碍损伤修复,导致牙齿脱落,最终影响整体健康。众所周知,转化生长因子-β1 (TGF-β1)具有再生特性,在干细胞治疗中是一种功能性的旁分泌因子,但其在调节PDLSC活性中的确切作用仍存在争议,且尚不清楚。目的阐明TGF-β1在PDLSC衰老中的作用,并确定其潜在的分子机制,从而促进我们对年龄相关性牙周病的认识,为制定针对性的治疗策略提供依据。方法采用空间转录组学方法,研究Tgfb1 mRNA在小鼠颌骨组织中的表达,重点研究其在牙周组织中的分布。伪时间分析用于评估表达模式和推断时间动态。我们以人PDLSCs为模型,研究TGF-β1信号传导的作用,通过检测DNA甲基化、衰老表型、细胞周期阻滞和潜在的信号传导途径。结果空间转录组分析显示Tgfb1在牙周组织中表达富集,并有上调的趋势。在人PDLSCs中,TGF-β1处理诱导衰老表型,其标志是G2期细胞周期停滞和活性氧(ROS)积累增加。在机制上,TGF-β1通过DNA甲基化介导的PRKAG2(编码AMPKγ2的基因)沉默触发ROS产生,导致ROS积累、DNA损伤和ATM信号激活。重要的是,用n -乙酰-l-半胱氨酸(NAC)抑制ROS或用地西他滨逆转PRKAG2表观遗传沉默通过抑制ATM信号减轻PDLSC衰老。我们的工作首次展示了小鼠颌骨组织的空间解析转录组景观,揭示了DNA甲基化是TGF-β1诱导PDLSC衰老的重要机制。这些发现阐明了TGF-β1信号、ROS产生和表观遗传调控之间先前未被认识到的联系,为开发基于干细胞的治疗方法来减轻与年龄相关的牙周病和改善全身健康提供了有希望的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Multi-omics approach reveals TGF-β signaling-driven senescence in periodontium stem cells
Introduction
The periodontal ligament (PDL), a dynamic connective tissue that anchors teeth to the alveolar bone, enables tooth retention and facilitates continuous turnover. The integrity of the periodontium is maintained by periodontal ligament stem cells (PDLSCs), whose dysfunction and senescence with age can disrupt tissue homeostasis, hinder injury repair, and lead to tooth loss, ultimately impacting overall health. Transforming growth factor-β1 (TGF-β1) is known for its regenerative properties and as a functional paracrine factor in stem cell therapy, but its precise role in modulating PDLSC activity remains controversial and poorly understood.
Objectives
This study aims to clarify the role of TGF-β1 in PDLSC senescence and identify the underlying molecular mechanisms, thereby advancing our understanding of age-related periodontal diseases and informing the development of targeted therapeutic strategies.
Methods
We employed spatial transcriptomics to map Tgfb1 mRNA expression in murine jawbone tissues, focusing on its distribution in the periodontium. Pseudotime analysis was performed to assess expression patterns and infer temporal dynamics. Human PDLSCs were used as a model to investigate the effects of TGF-β1 signaling, with assays conducted to examine DNA methylation, senescence phenotypes, cell cycle arrest, and underlying signaling pathways.
Results
Spatial transcriptomic profiling revealed enriched Tgfb1 expression in the periodontium, with upregulation tendencies. In human PDLSCs, TGF-β1 treatment induced a senescent phenotype marked by G2 phase cell cycle arrest and increased reactive oxygen species (ROS) accumulation. Mechanistically, TGF-β1 triggered ROS production through DNA methylation-mediated silencing of PRKAG2, a gene encoding AMPKγ2, resulting in ROS accumulation, DNA damage, and ATM signaling activation. Importantly, inhibition of ROS with N-acetyl-l-cysteine (NAC) or reversal of PRKAG2 epigenetic silencing with decitabine mitigated PDLSC senescence by suppressing ATM signaling.
Conclusion
Our work presents the first spatially resolved transcriptomic landscape of murine jawbone tissues and uncovers DNA methylation as a crucial mechanism underlying TGF-β1-induced PDLSC senescence. These findings illuminate a previously unrecognized link between TGF-β1 signaling, ROS production, and epigenetic regulation, offering promising avenues for developing stem cell-based therapies to attenuate age-related periodontal diseases and improve systemic health.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


