亚基界面的物理化学特征及其在铁蛋白超家族自组装中的作用
IF 4.4
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
铁蛋白普遍存在,在铁稳态中起关键作用。它们被分为四个主要亚科:经典、细菌、细菌铁蛋白和Dps。它们的特征是具有四螺旋束结构域的亚基,并通过三个不同的区域相互作用——一个反平行界面(IntA)和两个垂直界面(IntB和IntC),共同形成一个笼状结构。在这里,我们试图描述跨亚族这些界面的可变性。我们发现IntA对于二聚体单元的组装至关重要,并且可能首先组装,其次是IntB和IntC的较小界面(以任何顺序),这对于笼形形成至关重要。这些界面的独特之处在于,虽然化学性质稳定,但它们的包裹较少,它们的大小介于蛋白质-蛋白质复合物和专性二聚体之间。本研究提供了对铁蛋白界面的详细探索,提供了对其组装及其作为载体蛋白的重要性的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
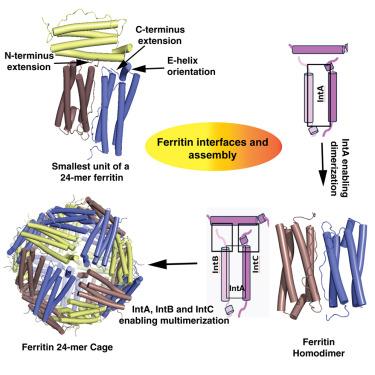
Physicochemical features of subunit interfaces and their role in self-assembly across the ferritin superfamily
Ferritins are ubiquitous and play a critical role in iron homeostasis. They are classified into four main subfamilies: classical, bacterial, bacterioferritin, and Dps. These are characterized by subunits with a four-helical bundle domain and interact through three distinct regions—one antiparallel interface (IntA) and two perpendicular interfaces (IntB and IntC), collectively forming a cage-like structure. Here, we attempt to characterize the variability of these interfaces across subfamilies. We found that IntA is essential for the dimeric unit assembly and is likely to assemble first, followed by the smaller interfaces of IntB and IntC (in any order), which are crucial for cage formation. These interfaces are unique in that they are less packed, although chemically stable, and their size lies between that of protein-protein complex and obligate homodimers. This study provides a detailed exploration of the ferritin interfaces, offering insights into their assembly and their importance as carrier proteins.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Structure
生物-生化与分子生物学
CiteScore
8.90
自引率
1.80%
发文量
155
审稿时长
3-8 weeks
期刊介绍:
Structure aims to publish papers of exceptional interest in the field of structural biology. The journal strives to be essential reading for structural biologists, as well as biologists and biochemists that are interested in macromolecular structure and function. Structure strongly encourages the submission of manuscripts that present structural and molecular insights into biological function and mechanism. Other reports that address fundamental questions in structural biology, such as structure-based examinations of protein evolution, folding, and/or design, will also be considered. We will consider the application of any method, experimental or computational, at high or low resolution, to conduct structural investigations, as long as the method is appropriate for the biological, functional, and mechanistic question(s) being addressed. Likewise, reports describing single-molecule analysis of biological mechanisms are welcome.
In general, the editors encourage submission of experimental structural studies that are enriched by an analysis of structure-activity relationships and will not consider studies that solely report structural information unless the structure or analysis is of exceptional and broad interest. Studies reporting only homology models, de novo models, or molecular dynamics simulations are also discouraged unless the models are informed by or validated by novel experimental data; rationalization of a large body of existing experimental evidence and making testable predictions based on a model or simulation is often not considered sufficient.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


