异八聚体block - 1相关配合物的结构与组装
IF 4.4
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
BORC (block - 1 -related complex)是一种异八聚体复合物,由8个螺旋状蛋白组成(BORCS1-8)。BORC通过募集ARL8控制溶酶体和突触囊泡的转运和定位。BORC组装和ARL8激活的结构机制尚不清楚。在此,我们重构并构建了这种杂八聚配合物的结构模型。我们发现BORC采用了一种由线圈组成的延伸棒状结构。两个半配合物,每个包含四个亚基,端到端连接形成全配合物。在每个半复合物中,BORCS1/4/6/8或BORCS2/3/5/7组装成类似的螺旋束。我们进一步研究了BORC是如何构建的,发现了BORCS1/2/3/5形成核心支架并招募其他亚基的分层组装过程。半复合物界面的突变产生两个半复合物。ARL8的关联可能需要BORC的适当组装,并且主要由BORCS5介导。这些结果为进一步认识BORC的生物学特性提供了指导。本文章由计算机程序翻译,如有差异,请以英文原文为准。
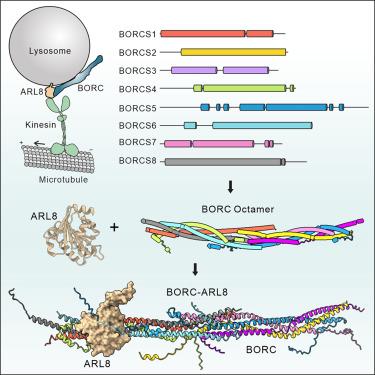
The structure and assembly of the hetero-octameric BLOC-one-related complex
BORC (BLOC-one-related complex) is a hetero-octameric complex, consisting of eight coiled-coil proteins (BORCS1–8). BORC controls lysosomal and synaptic vesicle transport and positioning by recruiting ARL8. The structural mechanisms underlying BORC assembly and ARL8 activation remain unclear. Here, we reconstitute and construct the structural model of this hetero-octameric complex. We find that BORC adopts an extended, rod-like structure made of coiled coils. Two hemicomplexes, each containing four subunits, are joined end-to-end to form the holocomplex. Within each hemicomplex, BORCS1/4/6/8 or BORCS2/3/5/7 assembles into similar helical bundles. We further study how BORC is built and discover a hierarchical assembly process in which BORCS1/2/3/5 forms the core scaffold and recruits other subunits. Mutations in the inter-hemicomplex interfaces result in two hemicomplexes. The association of ARL8 may require the proper assembly of BORC and is primarily mediated by BORCS5. These results provide guidance for further understanding of the biology of BORC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Structure
生物-生化与分子生物学
CiteScore
8.90
自引率
1.80%
发文量
155
审稿时长
3-8 weeks
期刊介绍:
Structure aims to publish papers of exceptional interest in the field of structural biology. The journal strives to be essential reading for structural biologists, as well as biologists and biochemists that are interested in macromolecular structure and function. Structure strongly encourages the submission of manuscripts that present structural and molecular insights into biological function and mechanism. Other reports that address fundamental questions in structural biology, such as structure-based examinations of protein evolution, folding, and/or design, will also be considered. We will consider the application of any method, experimental or computational, at high or low resolution, to conduct structural investigations, as long as the method is appropriate for the biological, functional, and mechanistic question(s) being addressed. Likewise, reports describing single-molecule analysis of biological mechanisms are welcome.
In general, the editors encourage submission of experimental structural studies that are enriched by an analysis of structure-activity relationships and will not consider studies that solely report structural information unless the structure or analysis is of exceptional and broad interest. Studies reporting only homology models, de novo models, or molecular dynamics simulations are also discouraged unless the models are informed by or validated by novel experimental data; rationalization of a large body of existing experimental evidence and making testable predictions based on a model or simulation is often not considered sufficient.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


