XPR1调控磷酸盐稳态的研究进展
IF 10.1
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
XPR1是人类唯一带注释的磷酸盐输出蛋白。最近的研究为其细胞功能提供了机制线索;3个假设非输出机制调节磷酸盐稳态,而6个提供高分辨率低温电镜数据支持由细胞内磷酸盐水平控制的真正的磷酸盐通道机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
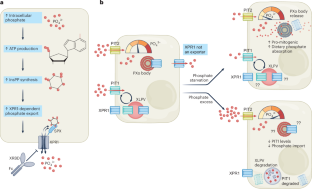

Insights into phosphate homeostasis regulation by XPR1
XPR1 is the only annotated phosphate exporter protein in humans. Recent studies provide mechanistic clues to its cellular function; three posit non-export mechanisms to regulate phosphate homeostasis, while six present high-resolution cryo-EM data supporting a bona fide phosphate channel mechanism controlled by intracellular phosphate levels.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


