双功能添加剂叔丁胺与镍、可见光的交叉偶联反应
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
过渡金属催化在复杂分子的合成中起着至关重要的作用,其中配体和碱基在优化交叉偶联反应中起着关键作用。尽管在配体设计和碱基选择方面取得了进步,但在这些成分之间实现有效的协同作用仍然具有挑战性。我们在这里提出了一种采用叔丁胺作为成本效益高的双功能添加剂,作为碱和配体的镍催化光氧化还原反应的一般方法。这种方法被证明是有效的C-O和C-N成键反应与各种亲核试剂,包括酚,脂肪醇,苯胺,磺胺,亚砜亚胺和亚胺。值得注意的是,该方案在生物分子衍生化中具有显著的适用性,并促进了顺序的一锅功能化。光谱研究揭示了动态催化体系的鲁棒性,而结构-反应性关系的阐明表明了亲核试剂和亲电试剂的计算分子性质如何与反应性能相关,为有效预测反应结果提供了基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
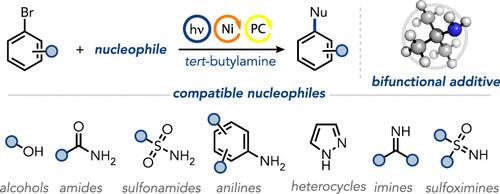
Cross-Coupling Reactions with Nickel, Visible Light, and tert-Butylamine as a Bifunctional Additive
Transition metal catalysis is crucial for the synthesis of complex molecules, with ligands and bases playing a pivotal role in optimizing cross-coupling reactions. Despite advancements in ligand design and base selection, achieving effective synergy between these components remains challenging. We present here a general approach to nickel-catalyzed photoredox reactions employing tert-butylamine as a cost-effective bifunctional additive, acting as the base and ligand. This method proves effective for C–O and C–N bond-forming reactions with a diverse array of nucleophiles, including phenols, aliphatic alcohols, anilines, sulfonamides, sulfoximines, and imines. Notably, the protocol demonstrates significant applicability in biomolecule derivatization and facilitates sequential one-pot functionalizations. Spectroscopic investigations revealed the robustness of the dynamic catalytic system, while elucidation of structure–reactivity relationships demonstrated how computed molecular properties of both the nucleophile and electrophile correlated to reaction performance, providing a foundation for effective reaction outcome prediction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


