冷冻诱导α-突触核蛋白组装成稳定微球制备光诱导货物释放系统
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
以淀粉样蛋白α-突触核蛋白(αS)为原料,通过冷冻诱导蛋白自组装制备了稳定的空心微球(MSs)。这个组装过程包括三个步骤:快速冷冻以形成αS低聚物的球形蛋白质凝聚体,冷冻退火以在凝聚物上形成外壳,冷冻干燥以通过三维(3D)咖啡渍效应形成内部腔。在冷冻退火过程中产生的结壳是一种β-薄片介导的蛋白质结构,被认为是在蛋白质-冰界面的准液体层产生的,因此有助于室温下MSs在水溶液中的稳定性,而无需任何额外的表面稳定。MSs在加热到70℃时转化为淀粉样纤维凝聚物,通过离心膜过滤装载药物。此外,用海藻酸铁层包埋金纳米颗粒(AuNPs)来屏蔽MSs,以防止过早泄漏和控制药物释放。这利用了AuNPs的光热效应,导致药物和热量之间的联合细胞毒性。因此,含有αS和AuNPs的载药MSs可以作为具有化学和物理抗癌作用的光可控药物传递系统。本文章由计算机程序翻译,如有差异,请以英文原文为准。
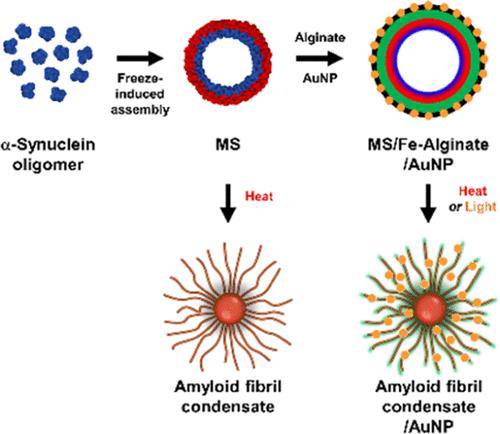
Freeze-Induced Protein Assembly of α-Synuclein into Stable Microspheres to Fabricate Light-Induced Cargo Release Systems
Stable hollow-type microspheres (MSs) have been fabricated using α-synuclein (αS), an amyloidogenic protein, via freeze-induced protein self-assembly. This assembly process involves three steps: rapid freezing to form spherical protein condensates from αS oligomers, frozen annealing to form a crust on the condensate and freeze-drying to create an interior lumen via the three-dimensional (3D) coffee-stain effect. The crust produced during the frozen-annealing step is a β-sheet-mediated protein structure that is presumed to be created at the quasi-liquid layer of the protein–ice interface and thus contributes to the stability of MSs in aqueous solutions at room temperature without any additional surface stabilization. MSs transform into amyloid fibril condensates when heated to 70 °C, and the drug is loaded via centrifugal membrane filtration. Additionally, the MSs were shielded with an iron-alginate layer embedded with gold nanoparticles (AuNPs) to prevent premature leakage and to control drug release. This takes advantage of the photothermal effect of AuNPs, resulting in combined cytotoxicity between the drug and heat. Therefore, drug-loaded MSs comprising αS and AuNPs can be suggested as light-controllable drug delivery systems that exhibit chemical and physical anticancer therapeutic effects.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


