使用多组学方法揭示PFOA毒性对滨海Zostera marina的影响:从生长,生理,转录组学和代谢组学特征的见解
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
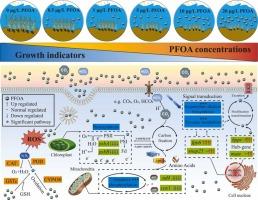
全氟辛酸(PFOA)是一种以其持久性、抗降解性和毒性而闻名的人为有机污染物,已引起人们对其潜在生态影响的严重关注。海苔是温带近海地区常见的沉水海草物种,对污染物胁迫具有高度敏感性。然而,PFOA对Z. marina的影响尚不清楚。在本研究中,将Z. marina暴露于不同浓度(0、0.5、1、5、10和20 μg/L)的PFOA环境中14天。随后,我们评估了生存率、生长模式、生理指标、转录组谱和代谢组学特征。结果显示PFOA在海参组织中呈剂量依赖性积累,并有明显的生长抑制作用。此外,PFOA暴露导致光合色素含量显著降低(IBRv2指数为2.78 ~ 10.29),酶活性显著升高(IBRv2指数为2.90 ~ 8.96)。转录组学分析鉴定出1511个差异表达基因,这些基因与11个主要受PFOA暴露影响的KEGG通路相关。加权基因共表达网络分析强调了羟基苯基丙酮酸还原酶(hppr)基因在抗氧化防御机制和pfoa诱导的应激解毒过程中的重要作用。代谢组学鉴定出412种差异表达的代谢物,主要由类黄酮、有机酸和脂类组成。综上所述,PFOA暴露导致光合作用和能量代谢相关基因表达下调,同时也影响代谢物合成。Z. marina对PFOA胁迫的反应包括调节细胞骨架动力学和信号转导途径,以及激活一系列基因和代谢物来启动防御机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Unraveling the impact of PFOA toxicity on Zostera marina using a multi-omics approach: Insights from growth, physiological, transcriptomic, and metabolomic signatures
Perfluorooctanoic acid (PFOA), an anthropogenic organic pollutant known for its persistence, resistance to degradation, and toxicity, has raised significant concerns about its potential ecological impacts. Zostera marina, a common submerged seagrass species in temperate offshore areas, is highly vulnerable to pollutant stressors. However, the impact of PFOA on Z. marina remains unclear. In this study, Z. marina was exposed to different concentrations of PFOA (0, 0.5, 1, 5, 10, and 20 μg/L) for 14 days. We subsequently assessed survival rates, growth patterns, physiological indices, transcriptomic profiles, and metabolomic characteristics. The results revealed dose-dependent PFOA accumulation in Z. marina tissues and significant growth inhibition. Furthermore, exposure to PFOA resulted in a significant reduction in photosynthetic pigment content (IBRv2 indices: 2.78–10.29) and elevated enzyme activity (IBRv2 indices: 2.90–8.96). Transcriptomic analysis identified 1511 differentially expressed genes associated with 11 KEGG pathways predominantly affected by PFOA exposure. Weighted gene co-expression network analysis highlighted the crucial role of the hydroxyphenylpyruvate reductase (hppr) gene in antioxidant defense mechanisms and detoxification processes against PFOA-induced stress. Metabolomics identified 412 differentially expressed metabolites, mainly consisting of flavonoids, organic acids, and lipids. In summary, PFOA exposure resulted in the down-regulation of gene expression related to photosynthesis and energy metabolism while also affecting metabolite synthesis. The response of Z. marina to PFOA stress involves modulation of the cytoskeletal dynamics and signal transduction pathways, as well as activation of a suite of genes and metabolites to initiate defense mechanisms.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


