配体调谐铝基金属有机骨架上锂离子吸附和同位素分离的机理研究
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
锂同位素分离是一项在核能和先进材料科学中有着广泛应用的关键技术。本研究探讨了通过改变有机配体合成的铝基金属有机骨架(Al-MOFs),特别是MIL-53、DUT-4、DUT-5、MIL-120和MIL-122的锂吸附和同位素分离能力。其中MIL-120表现出最高的锂吸附能力,在25 °C时,MIL-120的锂吸收量最大,为141.4 mg·g−1,由物理吸附机制驱动。同时,DUT-5在锂同位素分离方面表现优异,单级分离因子(α)为1.026,δ (7Li)值为−18.03。吸附热力学结果表明,MIL-120和MIL-122为吸热吸附,吸附量为ΔHθ = 58.96 kJ·mol−1,而MIL-53、DUT-4和DUT-5为化学吸附,吸附量为ΔHθ = 184.68 kJ·mol−1。结构和热重分析显示了不同的脱水和结合行为,MIL-120促进Li(H2O)4+配合物的表面吸附,DUT-5通过Li - o相互作用促进化学吸附。密度泛函理论(DFT)计算证实,与氧位的静电相互作用促进了MIL-120对锂的吸附,而DUT-5通过部分脱水形成更强的Al-O-Li键。材料表现出优异的稳定性,经过5次再生循环后,吸附容量保持在90% %以上。这些发现突出了Al-MOFs在锂同位素分离和回收技术领域的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
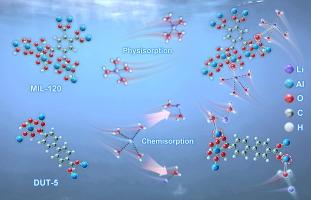
Mechanistic insights into lithium-ion adsorption and isotope separation on ligand-tuned aluminum-based metal–organic frameworks
Lithium isotope separation is a crucial technology with applications in nuclear energy and advanced material science. This study explores the lithium adsorption and isotope separation capabilities of aluminum-based metal–organic frameworks (Al-MOFs), notably MIL-53, DUT-4, DUT-5, MIL-120, and MIL-122, synthesized by altering organic ligands. Among them, MIL-120 demonstrated the highest lithium adsorption capacity, with a maximum uptake of 141.4 mg·g−1 at 25 °C, driven by physisorption mechanisms. At the same time, DUT-5 excelled in lithium isotope separation, achieving a single-stage separation factor (α) of 1.026 and a δ (7Li) value of −18.03. Adsorption thermodynamics indicated that the process for MIL-120 and MIL-122 was endothermic with ΔHθ = 58.96 kJ·mol−1, while MIL-53, DUT-4, and DUT-5 followed a chemisorption pathway (ΔHθ = 184.68 kJ·mol−1). Structural and thermogravimetric analyses revealed distinct dehydration and binding behaviors, with MIL-120 facilitating surface adsorption of complexes and DUT-5 promoting chemisorption through Li–O interactions. Density functional theory (DFT) calculations confirmed that electrostatic interactions with oxygen sites facilitate lithium adsorption of MIL-120, whereas DUT-5 forms stronger Al-O-Li bonds via partial dehydration. The materials exhibited excellent stability, retaining over 90 % of adsorption capacity after five regeneration cycles. These findings highlight the potential of Al-MOFs in the field of lithium isotope separation and recovery technologies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


