d -甘露糖介导的代谢途径维持精子功能和受精的分子特征
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
哺乳动物单次射精中的精子表现出显著的异质性,只有一小部分具有成功受精所需的分子特征。确定这些高生育能力精子的决定性特征仍然是一个悬而未决的问题。目的探讨小鼠和人类精子受精潜能的分子标记及其机制,重点研究d -甘露糖在精子受精中的作用。方法采用流式细胞术、生化检测和免疫荧光法分析精子形态和功能。多组学分析,包括蛋白质组学、代谢组学和脂质组学,被用于识别不同的分子特征。采用药物干预来验证关键通路,特别是Akt/mTOR信号通路的作用。结果鞭毛较长的精子在小鼠和人体内表现出更强的运动性、线粒体活性和受精潜力。多组学分析揭示了高生育能力精子的独特分子特征,其特征是特定的蛋白质、脂质和代谢物。值得注意的是,d -甘露糖补充剂通过激活Akt/mTOR通路,增强了精子活力和受精能力,即使在弱精子精子中也是如此。d -葡萄糖或ATP的补充没有复制这种效果。从机制上讲,d -甘露糖绕过糖酵解速率限制步骤,增加ATP的产生并促进线粒体和顶体的完整性。结论本研究确定了具有受精能力的精子的关键分子特征,并强调了d -甘露糖是精子质量和功能的新型调节剂。这些发现为精子生物学提供了有价值的见解,并为治疗男性不育症和优化辅助生殖技术提出了创新的治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
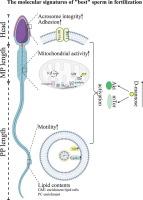
D-Mannose-Mediated metabolic pathways sustain the molecular signatures of sperm function and fertilization
Introduction
Mammalian sperm within a single ejaculate exhibit significant heterogeneity, with only a subset possessing the molecular characteristics required for successful fertilization. Identifying the defining traits of these high-fertility sperm remains an open question.
Objectives
To elucidate the molecular markers and mechanisms underlying the fertilization potential of sperm in both mice and humans, with a focus on the role of D-mannose.
Methods
Sperm morphology and functionality were analyzed using flow cytometry, biochemical assays, and immunofluorescence. Multi-omics analyses, including proteomics, metabolomics, and lipidomics, were conducted to identify distinct molecular signatures. Pharmacological interventions were employed to validate the role of key pathways, particularly Akt/mTOR signaling.
Results
Sperm with longer flagella demonstrated enhanced motility, mitochondrial activity, and fertilization potential in both mice and humans. Multi-omics analyses revealed distinct molecular profiles in high-fertility sperm, characterized by specific proteins, lipids, and metabolites. Notably, D-mannose supplementation enhanced sperm motility and fertilization capacity, even in asthenozoospermic sperm, by activating the Akt/mTOR pathway. This effect was not replicated by D-glucose or ATP supplementation. Mechanistically, D-mannose bypassed glycolytic rate-limiting steps, increasing ATP production and promoting mitochondrial and acrosomal integrity.
Conclusion
This study identifies key molecular signatures of fertilization-competent sperm and highlights D-mannose as a novel modulator of sperm quality and function. These findings provide valuable insights into sperm biology and propose innovative therapeutic strategies for treating male infertility and optimizing assisted reproduction technologies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


