铝诱导的正交SnO2和氧空位协同促进二羟基丙酮生成乳酸
IF 6.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
正交SnO2是SnO2的亚稳晶相。其合成难度大,条件苛刻,制约了其进一步利用和开发。在温和的条件下,采用易共沉淀法合成了正交SnO2,其中Al诱导了正交SnO2的形成并稳定了其存在。研究了正相SnO2在水热催化二羟基丙酮制乳酸中的作用机理。结果表明:在Al-Sn复合金属氧化物中,正晶型SnO2含量占SnO2总量的42.3%,且存在大量的氧空位;正交SnO2和氧空位协同促进弱Lewis酸位点的形成。弱Lewis酸位点显著提高了乳酸选择性,乳酸收率为97.21%。此外,该催化剂表现出优异的稳定性,第五次使用时乳酸产率达到89%。本研究提供了一种合成正交SnO2的新方法,并为其在水热催化中的应用提供了新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
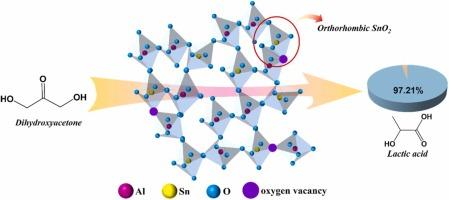
Al-induced orthorhombic SnO2 and oxygen vacancies synergistically promote lactic acid production from dihydroxyacetone
Orthorhombic SnO2 is a metastable crystalline phase of SnO2. Its synthesis is too difficult to require harsh conditions, inhibiting its further utilization and development. In this work, orthorhombic SnO2 has been synthesized by facile co-precipitation under mild conditions, in which Al induces the formation of orthorhombic SnO2 and stabilizes its presence. The action mechanism of orthorhombic SnO2 in the hydrothermal catalyzed conversion of dihydroxyacetone to lactic acid was investigated. The results show that the content of orthorhombic SnO2 is 42.3 % of the total SnO2 in the Al-Sn composite metal oxides, and a large number of oxygen vacancies occur. Orthorhombic SnO2 and oxygen vacancies synergistically promote the formation of weak Lewis acid sites. The weak Lewis acid sites significantly improved the lactic acid selectivity, leading to a lactic acid yield of 97.21 %. In addition, the catalyst exhibits excellent stability with 89 % lactic acid yield obtained at the fifth use. The present work provides a novel method for synthesizing orthorhombic SnO2 and offers new insights into its application in hydrothermal catalysis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Alloys and Compounds
工程技术-材料科学:综合
CiteScore
11.10
自引率
14.50%
发文量
5146
审稿时长
67 days
期刊介绍:
The Journal of Alloys and Compounds is intended to serve as an international medium for the publication of work on solid materials comprising compounds as well as alloys. Its great strength lies in the diversity of discipline which it encompasses, drawing together results from materials science, solid-state chemistry and physics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


